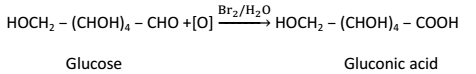Chemical properties of Glucose
Chemical properties:- Glucose contains one primary and four secondary hydroxyl groups and one aldehydic group. Glucose shows the reactions of all these groups. Some simple reactions are:-
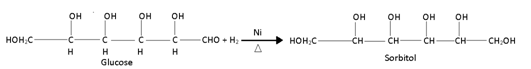
(1) Reduction:-
(i) Glucose is reduced to sorbitol, a hexahydric alcohol in presence of Ni as catalyst:
(ii) Reduction with HI:- Glucose on prolonged heating with HI gives n – hexane.
HOCH2 – (CHOH)4 – CHO + HI H3C – CH2 – CH2 – CH2 – CH2 – CH3
Glucose n – Hexane
(2) Oxidation:-
(i) Glucose on oxidation with mild oxidizing agent such as bromine water gives gluconic acid:
(ii) Glucose is oxidized with Tollen’s regent and Fehling’s solution to give Silver mirror and Red Ppt. Respectively:
HOCH2 – (CHOH)4 – CHO + 2[Ag(NH3)2]+ OH- → HOCH2 – (CHOH)4 – COOH + 2Ag + 4NH3 + H2O
Tollen’s Reagent Silver mirror
HOCH2 – (CHOH)4 – CHO + 2Cu2+ + 5OH- → HOCH2 – (CHOH)4 – COO + Cu2O + H2O
(Fehling’s solution) Red Ppt.
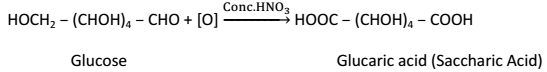
(iii) Strong oxidizing agent such as Conc. HNO3 oxidizes glucose to glucaric acid (Saccharic Acid):
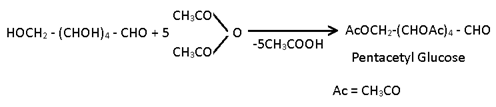
(3) Acylation:- When glucose is reacted with acetic anhydride in presence of H2SO4 a penta acetyl derivative of glucose is obtained.
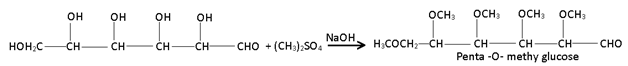
(4) Ether formation:- Glucose reacts with dimethyl sulphate in the presence of alkali to form penta – O – methyl derivative.
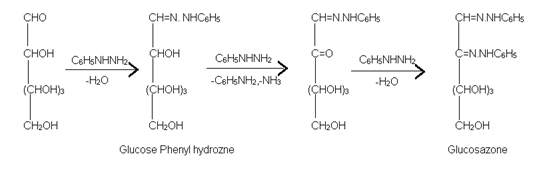
(5) Formation of Osazone:- Glucose on reaction with phenyl hydrazine forms phenyl hydrazone. However, when warmed with excess phenyl hydrazine, each glucose molecule reacts with three molecules of phenylhydrazine and yield osazone.
(6) Reaction with Hydroxylamine:- Glucose monoxime is formed.
HOCH2 – (CHOH)4 – CHO + H2NOH → HOCH2 – (CHOH)4 – CH = N – OH + H2O
Glucose Glucose monoxime
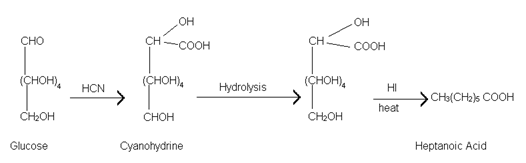
(7) Formation of Cyanohydrine:- Glucose on reaction with hydrogen cyanide gives cyanohydrine. Hydrolysis of cyanohydrine and reduction of acid obtained with hydrogen iodide yields heptanoic acid.
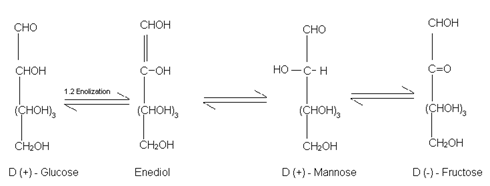
(8) Reaction with NaOH (Labry de Bruyn Van Ekenstein Rearrangement):- When treated with conc. NaOH, it first turns yellow, then brown and finally a brown resinous mass is obtained. However when treated with Dilute NaOH, it undergoes reversible isomesisation to form and equilibrium mixture of D – glucose, D – fructose and D – mannose via 1, 2 enolization:
This rearrangement is called Lobry De Bruyn Van Ekenstein Rearrangement.
 SureDen
SureDen