Cell and Energy cycle
The complex organic compounds (molecules) such as Carbohydrates, Proteins, Nucleic, Acids, Lipids, Hormones, Vitamins etc., Which form the basis of life, are called Biomolecules. These are essential for building up living organisms and are required for their growth and maintenance. These biomolecules take part in the biochemical processes occurring in the living organisms and are essential for the substance of life. These processes take place mostly at a pH = 7, temperature = 37° C and are highly selective and fast.
Cell and Energy cycle (Cellular Energetics):-
The cell is the fundamental, primitive unit of life. Cells are packets of chemicals essential for life. They have the ability to grow and divide to produce cells. The cells form organism as:
Cells → Tissues → Organs → Organism
A living cell contains about 50 elements. The most abundant 11 elements in living organisms are O, C, H, N, Ca, P, K, S, Cl, Na and Mg. The most abundant substance is water (70% of weight) along with other organic compounds. These compounds can be divided into two classes.
(i) Small molecules having molar mass in the range of 100 – 1000 and containing up to 30 carbon atoms.
(ii) The macromolecules having high molar masses e.g. Carbohydrates, proteins, nucleic acids etc.
A living system involves a set of complex reactions which is known as Metabolism. The process of metabolism occurs in two ways.
Anabolism:- The set of chemical reactions by which the various molecules of the cell are synthesized is called the anabolism.
Catabolism:- The set of reactions which involves the degradation of complex organic molecules into smaller ones of ultimately to CO2 and H2O with the liberation of energy is called Catabolism.
The Principle of thermodynamic feasibility is applicable to cellular reactions also. The phenomenon of energy changes occurring in the Biological reactions is called Cellular Energies. Most of Catabolic reactions occurring in a cell are thermodynamically feasible as ΔG is –ve (ΔG < 0) for these reactions. Such reactions for which ΔG is –ve are called Exergonic Reactions. On the other hand, most of the anabolic reactions in a cell are not thermodynamically feasible as ΔG positive is (ΔG > 0) for these reactions. Such reactions for which ΔG is positive are called Endergonic Reactions. Thus the anabolic process is always coupled with catabolic process in such a way that ΔG for both the reactions is negative. In most of the cellular reactions the energy releasing reaction (ΔG < 0) is the hydrolysis of Adenosine Triphosphate (ATP).
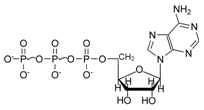
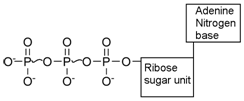
Adenosine Triphosphate (ATP):- The energy currency of the cell:- ATP is the compound which often acts as a link between energy requiring and energy releasing process in a cell. In other words, ATP functions as the carrier of chemical energy form the energy releasing to energy requiring process in the cell. The structure of ATP is:
ATP is an energy rich compound. The bond between the ribose sugar and the first phosphoric acid unit is an ordinary low energy, phosphate bond (i.e. energy is consumed when this bond is broken). But oxygen – phosphate bonds between the two phosphoric acid residues (shown by ~) are high-energy phosphate bond (i.e. energy is released when this bond is broken). There are four oxygen atoms with negative charge which repel each other and make it energy rich. In ATP energy is easily transferred, transformed and stored. Hence ATP is called the energy currency of the cell.
 SureDen
SureDen