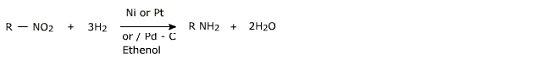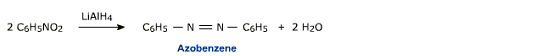Chemical Properties of Nitro Compounds
Reduction of nitroalkane
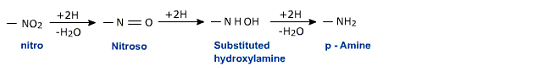
Nitro compounds can be reduced to primary amines under a variety of conditions. The various reduction stages of the nitro group are given below:
The final product depends on the nature of the reducing agent as well as the pH of the medium.
i) Catalytic reduction
The nitro group is easily reduced by catalytic hydrogenation using Pd/C catalyst in ethanol.
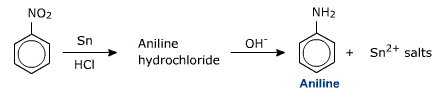
ii) Reduction by metal in acidic solutions
Metals (Fe, Sn and Zn) and HCl are sued for reducing a nitro group (-NO2) to an amino group (-NH2).
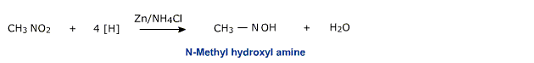
iii) Reduction in neutral medium
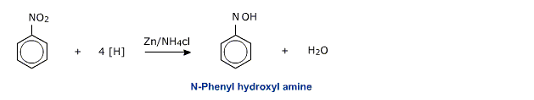
Zinc dust and ammonium chloride convert nitro benzene to the corresponding hydroxylamine.
The hydroxyl amines on warning with ammoniacal silver nitrate solution (Tollen's reagent) get oxidised to nitroso compound and reduce the Tollens reagent to metallic silver. This reaction is used as a test for nitro compounds and is called Baker - Mullikens' test.
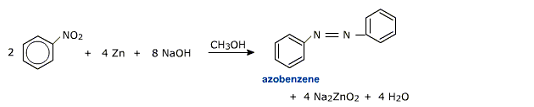
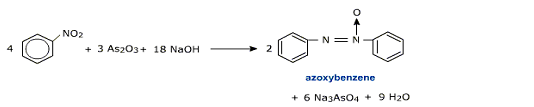
iv) Reduction in alkaline medium
Depending upon the nature of the reducing agent, nitrobenzene forms different products.
v) Reduction with LiAlH4
Aliphatic nitro compounds are reduced to p-amines with LiAlH4.
Aromatic nitro compounds on reduction with LiAlH4 give azo compounds.
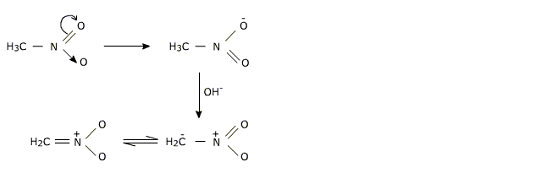
Acidic nature of alpha hydrogen atom
Alpha hydrogen atom in case of aliphatic nitro compounds becomes acidic due to electron withdrawing nature of nitro group.
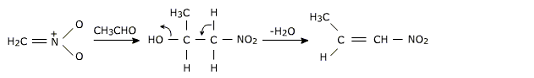
This nucleophic anion performs reactions with electrophiles such as acetaldehyde to form aldol condensation product which on dehydration gives unsaturated nitro compound.
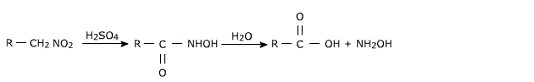
Hydrolysis of aliphatic nitro compounds
Primary aliphatic nitro compounds can be converted to aldehydes by treatment of their carbanion salts with sulphuric acid.
When primary nitro compounds are treated with H2SO4 without prior conversion to salts, they give carboxylic acids.
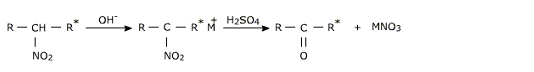
Secondary nitro alkanes hydrolyze with boiling HCl to give ketones and nitrous oxide.
 SureDen
SureDen