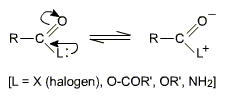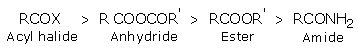Physical properties of Acid Derivatives
Structure of functional group in carboxylic acid derivatives
The structure of the functional groups in acyl halide, acid anhydride, ester and amide are similar to that of the carboxyl group. Due to the presence of lone pairs of electrons at the halogen, oxygen and nitrogen atoms, resonance is possible in these derivatives just like that in carboxylic acids.
The nature of the L group determines the relative electrophilic nature of the carbonyl carbon and thus the relative reactivity of the acyl derivatives.
All acid derivatives are polar molecules.
Physical properties
Being polar in nature, the acid derivatives have higher boiling points than hydrocarbons of comparable molecular masses.
Acid chlorides, anhydrides and esters have nearly the same boiling points as the aldehydes and ketones of comparable molecular masses. Their boiling points are lower than that of carboxylic acids of comparable molecular masses, due to the absence of hydrogen bonding in acid derivatives.
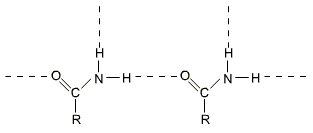
Primary amides have quite high melting points and boiling points because they form strong intermolecular hydrogen bonds.
Esters and amides of low molecular masses are fairly soluble in water due to formation of hydrogen bonds with water. The solubility in water decreases with increasing molecular mass and is negligible for compounds containing more than six carbon atoms.
All acid derivatives are soluble in usual organic solvents. Volatile esters have pleasant fruity smell. Acyl halides and anhydrides have sharp irritating odors and are lachrymatory (tear producing).
Reactivity of acid derivatives
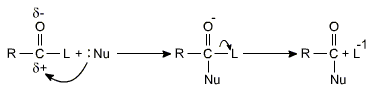
The reactions of carboxylic acids and their derivatives involve substitution of the group L with nucleophiles and are known as nucleophilic acyl substitution reaction.
Order of reactivity of the acid derivatives is
Carboxylic acids and their derivatives can be interconverted by nucleophilic acyl substitution reactions.
 SureDen
SureDen