Introduction
Introduction, structure of carbonyl group, nomenclature and isomerism in carbonyl compound
An aldehyde is either a functional group consisting of a terminal carbonyl group, or a compound containing a terminal carbonyl group. A carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. Aldehydes are named by IUPAC nomenclature by changing the suffix -e of the parent alkane (A non-aromatic saturated hydrocarbon with the general formula CnH2n+2) to -al.
The simplest member of the aldehyde group of organic compounds, formaldehyde, or methanol. Aldehyde has the general formula RCOH where the group R is any hydrocarbon.
Ketone is either the functional group characterized by a carbonyl group linked to two other carbon atoms or a compound that contains this functional group. Ketone is a class of chemical compounds contains the carbonyl group in which the carbon atom is covalently bonded to an oxygen atom.
Ketones are named by appending -one to the stem. Ketone has the general formula RCOR' where the groups R and R' may be the same or different, or incorporated into a ring.
Boiling point of aldehydes and ketones are somewhat higher than those of alkanes of comparable molecular mass due to dipole-dipole interactions between the opposite ends of the carbonyl group. However, their boiling points are lower than those of corresponding alcohols and carboxylic acids due to the absence of intermolecular H- bonding.
Structure of carbonyl group
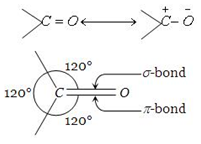
Carbonyl carbon atom is joined to three atoms by sigma bonds. Since these bonds utilise sp2-orbitals, they lie in the same plane and are 120° apart. The carbon-oxygen double bond is different than carbon-carbon double bond. Since, oxygen is more electronegative, the electrons of the bond are attracted towards oxygen. Consequently, oxygen attains a partial negative charge and carbon a partial positive
charge making the bond polar. The high values of dipole moment, 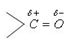
(2.3 – 2.8D) cannot be explained only on the basis of inductive effect and thus, it is proposed that carbonyl group is a resonance hybrid of the following two structures.
 SureDen
SureDen