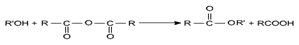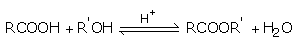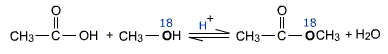Esters
Esters
RCOOR' are named after the corresponding carboxylic acids by replacing the ending -ic acid with -ate and preceding this with the name of the alkyl or aryl group attached to the oxygen atom.
Preparation
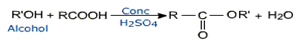
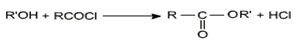
Esters are prepared by the acylation of alcohols or phenols.
The formation of esters is known as esterification.
Esterification of carboxylic acids with alcohols requires a mineral acid such as concentrated H2SO4 or HCl gas a catalyst.
(Fischer Esterification)
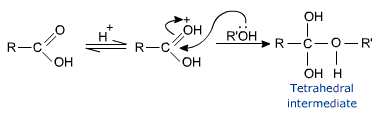
Mechanism of esterification of carboxylic acids
The esterification of carboxylic acids with alcohols is a kind of nucleophilic acyl substitution.
The first step is the protonation of the carbonyl oxygen, which activates the carbonyl group towards nucleophilic addition of the alcohol.
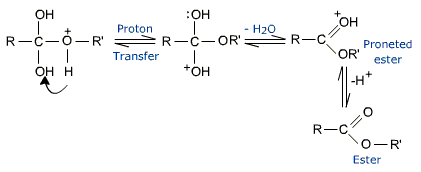
The tetrahedral intermediate, which is formed transfers a proton converting the hydroxyl group into 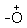 H2 group. This species
H2 group. This species 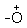 H2 is a better leaving group and is eliminated at a neutral water molecule.
H2 is a better leaving group and is eliminated at a neutral water molecule.
The protonated ester finally losses a proton to give the ester.
The above mechanism is supported by the using isotopically labeled methanol (CH3O18H) with acetic acid to give methyl acetate (having labeled oxygen) and water not containing any isotopic oxygen.
Esters of phenols are prepared by reversible acylation of phenols with acyl chloride or anhydrides rather than the reaction with carboxylic acid in which all the steps are reversible.
Reactions
Esters undergo typical nucleophilic acyl substitution reactions but are less reactive than acyl chlorides and anhydrides.
i) Hydrolysis
Esters hydrolyzed by boiling water slowly to carboxylic acids and phenols. The hydrolysis is accelerated in the presence of mineral acid on alkali.
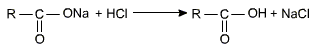
The alkaline hydrolysis is known as saponification. This is because esters with high molecular mass (C12 - C17) give soap on hydrolysis with a base. Soap on hydrolysis with a base. Soaps are sodium or potassium salts of Carboxylic acids with high molecular mass (C12 - C17). The carboxylic acid is obtained by acidification of the salt with mineral acid (H2SO4 or HCl).
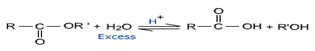
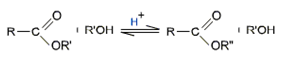
ii) Alcoholysis
Esters react with alcohols in the presence of an acid catalyst to undergo exchange of alcohol resides i.e., alkoxy parts. The equilibrium mixture consists of the reactants and a new ester and a new alcohol. The reaction involves nucleophilic acyl substitution of the alkoxy group of the ester with the alkoxy group of the alcohol and is known as trans esterification.
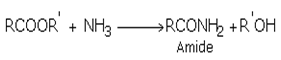
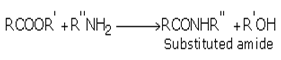
iii) Esters react with ammonia and amines to form amides
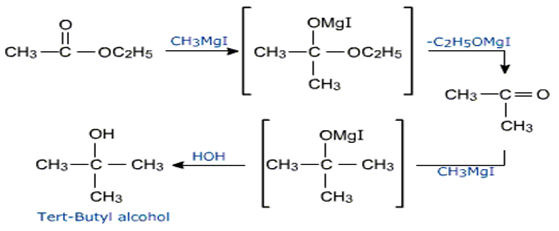
iv) Reactions of esters with Grignard's reagent gives tertiary alcohols.
First a ketone is formed which reacts further with Grignard reagent to give the tertiary alcohol.
(v) The acyl group of the ester is reduced with LiAlH4 (but not with NaBH4) to a primary alcohol.
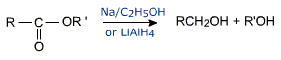 Catalytic hydrogenation of esters to alcohols is not easy unlike that of aldehydes and ketones. The reaction requires high temperature and pressure. The catalyst used is a mixture of oxides known as copper chromite. The alkoxy part of the ester gives the corresponding alcohol as by product.
Catalytic hydrogenation of esters to alcohols is not easy unlike that of aldehydes and ketones. The reaction requires high temperature and pressure. The catalyst used is a mixture of oxides known as copper chromite. The alkoxy part of the ester gives the corresponding alcohol as by product.
 SureDen
SureDen