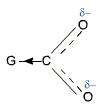
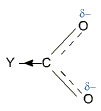
Effect of Substituents
Effect of substituents on the acidity of carboxylic acids
The factors, which increase the stability of carboxylate ion more than the carboxylic acids, increase the acidic strength of acid and the factors that decrease the stability of carboxylate ion decrease the acid strength.
Electron withdrawing groups such as halo group, -NO2, -CN etc increase the acidity of carboxylic acids. These electron withdrawing groups stabilize the carboxylate anion by dispersal of the negative charge and increase the strength of the acid.
Electron releasing groups like alkyl groups cause concentration of negative charge, destebalize the carboxylate anion and decrease the strength of the acid.
The more powerful and the more the number of such substituents and the closer they are to the carboxyl group, the greater is the inductive effect.
The FCH2COOH > ClCH2COOH > Br CH2COOH > ICH2COOH > CH2COOH.
The electronegativity and thus the electron withdrawing power of the halogen substituent and thus the strength of the acid decreases in the given order.
CH3CCOOH > Cl2CHCOOH > ClCH2COOH > CH3COOH
The number of electron withdrawing chloro group and thus the acid strength decreases in this order.
CH3CH2CH(Cl)COOH > CH3CHCl CH2 COOH > ClCH2CH2CH2COOH > CH3CH2CH2 COOH
The inductive effect decreases rapidly with increasing distance from the carboxylic group and so does the acid strength.
 SureDen
SureDen