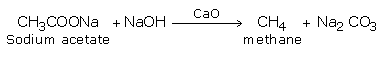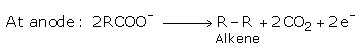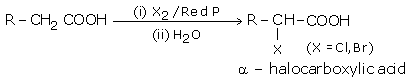Chemical properties of Carboxylic Acids
Chemical properties
Reduction
a) Partial reduction
Carboxylic acids are reduced to primary alcohols on treatment with lithium aluminium hydride or better with diborane. Here -COOH group is reduced to -CH2OH.
Sodium borohydride does not reduce the carboxyl group.
b) Complete reduction
Carboxylic acids are reduced to alkenes on reaction with HI in the presence of Phosphorus at about 500 K. Here -COOH group is reduced to -CH3.
RCOOH + 6HI RCH3 + 3H2O + 3I2
CH3 COOH + 6HI + 6HI CH3 CH3 + 2H2O + 3I2
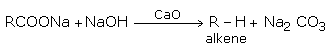
3. Decarboxylation
Sodium or potassium salts of carboxylic acids on heating with soda lime (NaOH and CaO) gives alkanes with one carbon less than the parent acids. (In this reaction while NaOH takes part in the reaction, CaO simply helps in fusion of NaOH)
Alkali metal salts of carboxylic acids undergo decarboxylation on electrolysis of their aqueous solutions and form hydrocarbons having twice the number of carbon atoms present in the alkyl group of the acid. This reaction is known as Kolbe's electrolysis.
RCOONa (aq) ⟶ RCOO- + Na+
At cathode: 2H2 O + 2e- ⟶ H2 + 2OH-
Substitution
i) Halogenation
Carboxylic acids having an a-hydrogen are halogenated at the a-position on treatment with chlorine or bromine in the presence of small amount of red phosphorus to give a-halocarboxylic acids. This reaction is known as Hell - Volhard Zelinsky reaction.
ii) Ring substitution in aromatic acids
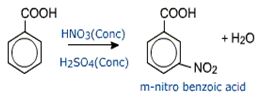
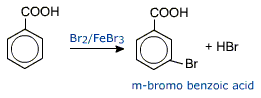
Aromatic carboxylic acids undergo electrophilic substitution reactions in which the carboxyl group is a deactivating and meta directing group.
Aromatic carboxylic acids, however do not undergo Friedel - Craft's reaction.
 SureDen
SureDen