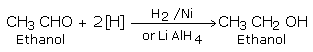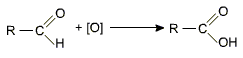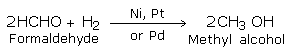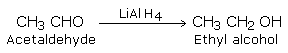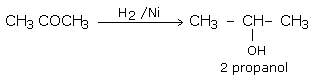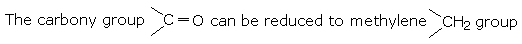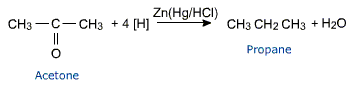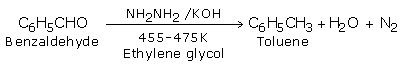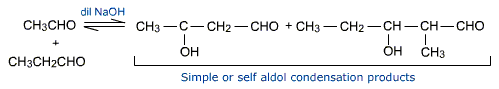Chemical Properties of Aldehydes and Ketones
Chemical properties of aliphatic aldehydes and ketones
ADDITION REACTIONS
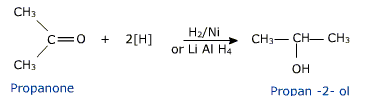
Aldehydes and ketones are reduced to the corresponding alcohols by
a) Addition of hydrogen in the presence of catalysts like finely divided platinum, palladium, nickel and ruthenium.
This method is called catalytic hydrogenation.
b) Treatment with chemical reagents such as sodium borohydride (NaBH4) or Lithium aluminium hydride (LiAlH4).
Aldehydes yield primary alcohols while ketones give secondary alcohols.
OXIDATION REACTIONS
Aldehydes differ from ketones in their oxidation reactions. Aldehydes are easily oxdised to carboxylic acids on treatment with common oxidising agents like nitric acid, potassium permanganate, potassium dichromate etc.
Aldehydes are easily oxidised to carboxylic acids containing the same number of carbon atoms, as in parent aldehyde.
The reason for this easy oxidation is the presence of a hydrogen atom on the carbonyl carbon, which can be converted into -OH group without involving the cleavage of any other bond. Thus even weak oxidising agents like bromine water, Ag+, Cu2+ etc are effective. As a result aldehydes act as strong reducing agent.
Ketones are not easily oxidised. Under vigorous condition, their oxidation involves carbon-carbon bond cleavage giving a mixture of carboxylic acids having lesser number of carbon atoms than the parent ketone.
These oxidation reactions can be used to distinguish aldehydes from ketones.
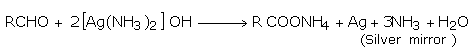
i) Aldehydes gives the Tollen's test on warming an aldehyde with freshly prepared ammoniacal silver nitrate solutions (Tollen's reagent) in a clean test tube in a water bath, a bright silver mirror is produced due to deposition of silver metal on the sides of the test tube. The reaction occurs in alkaline medium.
Ketones do not respond to this test.
AgNO3 + NH4OH ⟶ AgOH + NH4 NO3
AgOH + 2NH4OH ⟶ [Ag(NH3]2 OH + 2H2O
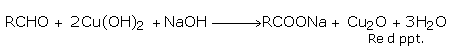
(ii) Aldehydes respond to the Fehlings' test.
Fehlings' solution is an alkaline solution of copper sulphate containing sodium potassium tartrate (Rochelle Salt) as a complexing agent. Aldehydes on warming with solution, give a red precipitate of cuprous oxide as a result of the redox reaction. Aromatic aldehydes give very poor results in this test.
Ketones do not reduce Fehling solution.
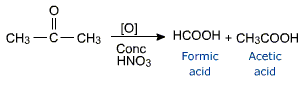
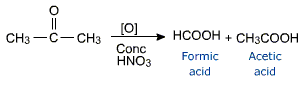
Oxidation of ketones
Ketones are oxidised only under vigorous conditions using powerful oxidising agents such as conc. HNO3, KMnO4/H2SO4, K2Cr2O7/H2SO4 etc. Oxidation of ketones involves cleavage of bond between carbonyl carbon and a-carbon on either side of keto group giving a mixture of carboxylic acids.
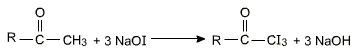
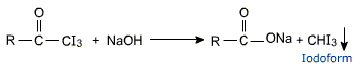
Haloform reaction
Ketones having at least one methyl group linked to the carbonyl carbon atom (i.e., methyl ketones) are oxidised by sodium hypohalite to sodium salts of carboxylic acids with one carbon atom less than that of the ketones. The methyl group is converted to haloform, which appears as a yellow precipitate. This oxidation does not affect a carbon-carbon double bond, if present in the molecule.
2NaOH + I2 ⟶ NaI + NaOI + H2O
This test is shown by acetaldehyde as well.
REDUCTION REACTIONS
Aldehydes and ketones can be reduced to a variety of compounds under different conditions with different reducing agents.
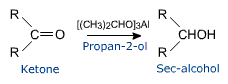
Reduction to alcohols
Aldehydes and ketones on mild reduction give primary and secondary alcohols respectively. This type of reduction is carried out either catalytically with H2 in the presence of Ni, Pt or Pd or chemically by LiAlH4(Lithium Aluminium Hydride) or NaBH4 (Sodium borohydride).
Ketones can also be reduced to secondary alcohols with aluminium isopropoxide in propan-2-ol solution.
This reaction is called Meerwein - Ponudorf reduction.
Reduction to hydrocarbon
resulting in the formation of alkanes by any of the following reagents.
a) Zinc amalgam and concentrated hydrochloric acid reduce and the reaction is called Clemmenson's reduction.
b) Wolff - Kishner reduction use by hydrazine followed by heating with potassium hydroxide in high boiling solvent ethylene glycol.
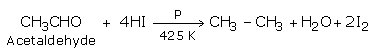
c) Hydro iodic acid and phosphorus reduce carbonyl compounds at 425 K.
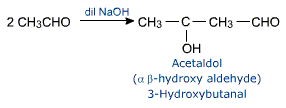
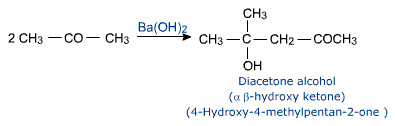
Aldol condensation
Two molecules of an aldehyde or a ketone having at least one a-hydrogen atom condense in the presence of a dilute acid to give a b-hydroxyaldehyde or b-hydroxy - ketone.
This reaction is called aldol condensation. The name is derived from the names of two functional groups, aldehyde and alcohol present in the product. Though ketones give ketols (a compound containing a keto and alcohol groups) the general name aldol condensation applies.
The aldol condensation involves the formation of bond between carbonyl carbon of one molecule and the a-carbon atom of the other molecule.
Mechanism of Reaction
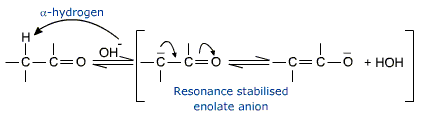
Acidity of a-hydrogen
The a-hydrogen in aldehydes and ketones is weakly acidic and may be removed by a base such as NaOH. The acidity of a-hydrogen is due to resonance staiblization of the conjugate base called the enolate anion.
[Enolate stands for the suffixes for the carbon-carbon double bond (ene) and the alcoholate group present.]
In the next step nucleophilic addition of the enolate anion occurs. The a-carbon of the enolate anion has considerably negative character and is thus nucleophilic. It adds to the carbonyl group of the unreacted aldehyde or ketone to give the aldol product.
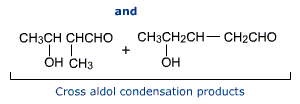
Cross aldol condensation
Aldol condensation of a mixture of two different aldehydes or / and ketones each containing an a-hydrogen gives a mixture of four products. In this reaction, each carbonyl compound produces the corresponding enolate anion. This enolate anion may add on to the compound with the same carbonyl group to give a simple aldol condensation products. The other two products arise when a different carbonyl compound may add on. This is cross aldol condensation and gives rise to the formation of cross aldol condensation products.
Example: A reaction between ethanol and propanal.
This cross aldol condensation has no synthetic value except when one of the carbonyl compounds has no a-hydrogen.
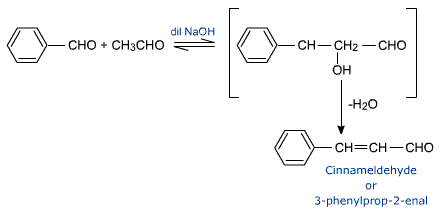
For e.g., in the reaction between benzaldehyde and acetoldehyde, the cross aldol product easily losses water molecule to give cinnamaldehyde.
In conclusion, all aldehydes and ketones which contain a-hydrogen atom undergo aldol condensation. Those which do not contain a-hydrogen like HCHO, C6H5CHO etc do not undergo this reaction.
However cross aldol condensation can occur between carbonyl compound having no a-H atom with aldehydes or ketones possessing a-H atom.
 SureDen
SureDen