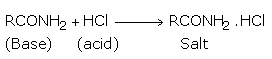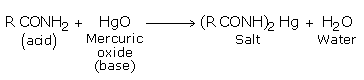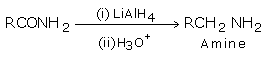Amides
Amides
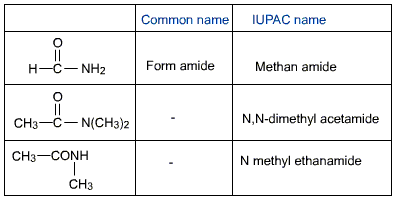
RCONH2 are named in the trivial system by replacing the ending -ic acid from the name of the corresponding acid with - amide. The IUPAC names are derived by replacing the ending -oic acid with -amide or carboxylic acid with carboxamide. The position of the substituent at the nitrogen atom, if any, is indicated by the letter N.
Amides are classified as primary, secondary and tertiary amides depending on whether none, one or two alkyl or aryl groups at attached to the nitrogen atom
RCONH2 - Primary
RCONHR' - Secondary
RCONR'R'' - Tertiary
Preparation
Amides are generally prepared by the reaction of acyl chlorides or anhydrides with ammonia or amines.
RCOCl + NH3 ⟶ RCONH2 + HCl
(RCO)2 O + 2NH3 ⟶ RCOONH4 + RCONH2
Carboxylic amines give ammonium carboxylates, which need to heated to high temperatures gives amides. Thus this method is not useful for laboratory preparation of amides. It is however used in the industrial preparation of amides.
RCOOH + NH3 ⟶ RCOO- NH+4 RCONH2 + H2O
Reactions
(i) Amphoteric character
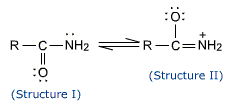
Amides are feeble bases. The lone pair of electrons on the nitrogen atom is responsible for the basic character. This lone pair of electrons on nitrogen atom is involved in resonance with the carbonyl group (structure II). Thus the electron pair of nitrogen is not easily available for protonation. Consequently the basic character is considerably decreased.
The basic character of the amide is illustrated in the following reaction with hydrochloric acid (an acid) to form a salt.
However, under suitable conditions, amides can also exhibit feeble acidic character. The amide (acting in the capacity of a acid) reacts with mercuric oxide (a base) to form mercury salt and water.
Thus amides are said to be amphoteric in nature as they exhibit both acidic and basic character.
(ii) Amides are hydrolyzed by aqueous solutions of mineral acids or alkalis to give carboxylic acids.
RCONH2 + NaOH RCOONa + NH3
(iii) Primary amides get dehydrated with phosphorous pentoxide to give nitriles.
(iv) On treatment with nitrous acid, primary amides give carboxylic acid and nitrogen gas. The volume of nitrogen can be measured to determine the amide quantitatively.
RCONH2 + HNO2 ⟶ RCOOH + N2 + H2O
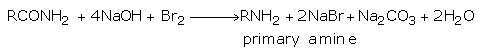
(v) When primary amides are treated with bromine in the presence of an alkali, a primary amine containing one carbon less than the amide is formed.
The reaction involves molecular management in which alkyl or aryl group migrates from the acyl carbon to nitrogen. This reaction is known as Hofmann Bromamide reaction and is useful for descending of series i.e., preparing a lower homologue from a higher one.
vi) Amides are reduced to amines with LiAlH4.
 SureDen
SureDen