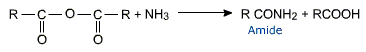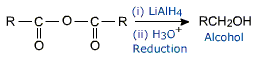Acid Anhydrides
Acid anhydrides
Acid anhydrides are called symmetrical when the two acyl groups are identical when the two acyl groups are identical and if the two acyl groups are different it is said to be unsymmetrical.
Symmetrical anhydrides of unsubstituted carboxylic acids are derived from the names of the carboxylic acids by replacing the word acid with anhydride.
Symmetrical anhydrides of substituted carboxylic acids are named by adding the prefix bis to the name to indicate that two identical acyl groups are present.
Unsymmetrical anhydrides are named by writing the names of the two acids alphabetically before the word anhydride.
Preparation
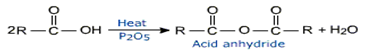
Acid anhydrides are considered to be derived from carboxylic acids by the removal of a molecule of water from two molecules of acid. So acid anhydrides can be prepared by heating carboxylic acid in the presence of P2O5 in dehydrating agent.
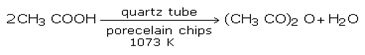
Industrially, acetic anhydride is prepared by heating acetic acid to 1073 K.
Acetic anhydride is a dehydrating agent
Symmetrical anhydrides are prepared from acids using a dehydrating agent like P2O5.
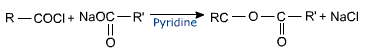
Asymmetrical and symmetrical anhydrides can be prepared by the reaction of acyl chlorides with sodium salts of carboxylic acids in the presence of pyridine.
Reactions
Acid anhydrides undergo reactions similar to acyl chlorides. They are good acylating agents.
They are hydrolyzed by water.
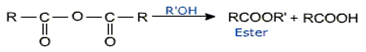
Alcoholysis
They form esters with alcohols and phenols.
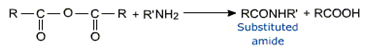
They form amides with ammonia and amines.
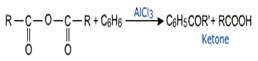
Friedel Craft's acylation reaction
Anhydrides are on the whole less reactive than acyl chlorides.
 SureDen
SureDen