Properties of Phenol
PHYSICAL PROPERTIES OF PHENOL :
PHYSICAL PROPERTIES OF PHENOL:
1) pure Phenols are either colourless liquids or deliquescent solids.
2) they usually turns to pink colour on exposure to air and light due to oxidation in air
3) phenols have peculiar smell and a strong corrosive action on skin.
4) the boiling point of phenol is much higher than corresponding aromatic hydrocarbons and halo arenes.higher boiling point is due to hydrogen bonding
5) soluble in water due to hydrogen bonding but less soluble in water as compared to alcohol‘
6) phenols are poisonous in nature bt act as an antiseptic and disinfectantant too.
CHEMICAL PROPERTIES OF PHENOL :
CHEMICAL PROPERTIES:
1)Ester formation:
2)Phenol in William son synthesis:
3)Reaction with ammonia:phenol is converted to aniline when heated with ammonia
4) Reaction with Zn dust:phenol when treated with zn gives benzene .
ACIDIC NATURE :
Acidic nature of phenol
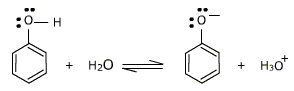
Phenols turn blue litmus red and react with metals liberating hydrogen. phenols are more acidic than alcohols but less then carboxylic acid.
Phenols behave as acids because of the presence of polar O-H group in them. They ionise in aqueous solutions to give H+ ions.
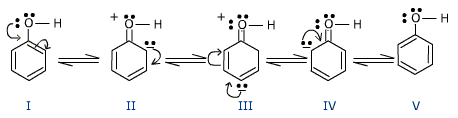
Phenols as well as phenoxide ion both are resonance stabilized and this is the reason for the high acidic character of pheno . The various contributing structures of phenol and phenoxide ion are given below:
such sructures are not possible in alcohol
 SureDen
SureDen