Preparation of Phenols
Introduction of phenols
A phenol contains -OH group(s) directly attached to an aryl carbon atom(s). The simplest phenol is hydroxybenzene also called phenol with formula C6H5OH. A phenolic compound hexachlorophene is a constituent of several mouthwashes, deodorant soaps and medicinal skin cleansers.
It must be noted that the aromatic compounds in which -OH group is not directly attached to benzene ring are not phenols but are called aromatic alcohols.
PREPARATION OF PHENOL :
Preparation of phenol
phenol was selected from coal tar by destructive distillation. The laboratory methods of preparation of phenols are:
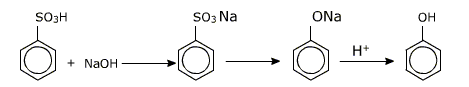
i) From aryl sulphonic acids (alkali fusion of sodium benzoate)
An aryl sulphonic acid yields the corresponding phenol on heating it with molten sodium hydroxide at 570 - 620 K. The sodium salt is obtained which is hydrolysed with acid to obtain free phenol.
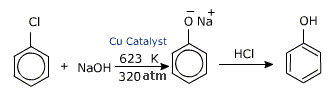
ii) From DOW ‘S PROCESS –HYDROLYSIS OF CHLORO BENZENE:
CHLORO benzene is heated at 350C UNDER high pressure with aq.sodium hydroxide. the reaction produces sodium phenoxide which on acidification yields phenol.
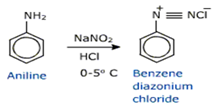
iii) Hydrolysis of diazonium salts
A diazonium salt is formed by treating an aromatic primary amine with nitrous acid (obtained from a mixture of NaNO2 and HCl).
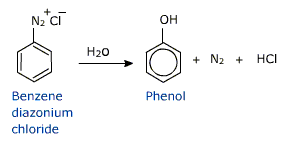
Diazonium salts are hydrolysed to phenols in presence of dilute acids.
 SureDen
SureDen