Physical Properties
Physical properties monohydric alcohols
Alcohols consist of two parts, an alkyl/aryl group and a hydroxyl group. The properties of alcohols and phenols are due to the -OH group.
The alkyl and aryl groups modify these properties.
1. The lower members of alcohols upto carbon number 11 are colourless, mobile liquid with a characteristic alcoholic smell and burning taste whereas higher alcohols are odourless and tasteless.
Higher alcohols having 12 or more carbon atoms are colourless waxy solids.
2. Solubility of alcohols:The first three members are completely miscible with water. The higher members are almost insoluble in water but are soluble in organic solvents like benzene, ether etc.
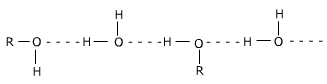
The solubility of lower alcohols is due to the existence of hydrogen bonds between water and polar -OH group of alcohol molecules.
The -OH group in alcohols and phenols contain a hydrogen bonded to an electronegative oxygen atom.
In addition, among the isomeric alcohols, the solubility increases with branching of chain. As the surface area of the non-polar part in the molecule decreases, the solubility increases.
3.Alcohol are toxic as well as neutral substances.
4. BOILING POINT: for alcohols it increases with increase in carbon chain .
Among isomeric alcohol yhe boiling point varies as in the order of
PRIMARY > SECONDARY > TERTIARY.
The reason for higher boiling point is due to inter molecular hydrogen bonding.
 SureDen
SureDen