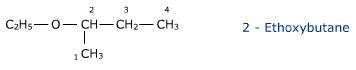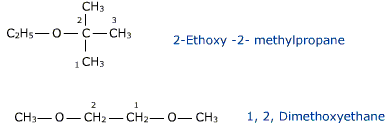Nomenclature of Ethers
Nomenclature of ethers
Common names of ethers are followed after the names of alkyl / aryl groups written as separate words in alphabetical order. The word ether is added at the end.
In case of simple ethers, the prefix di is attached before the name of the alkyl group.
Examples:
C2H5 - O - C2H5 is Diethyl ether
C6H5 - O - C6H5 is Diphenyl ether
According to the IUPAC nomenclature ethers are regarded as hydrocarbon derivatives in which a hydrogen atom is replaced by an alkoxy group – OR . The larger alkyl group forms the part of parent chain while lower alkyl group constitutes the alkoxy radical.ether have a bent structure and are dipolar in nature and hence has a net dipole moment.
Examples:
CH3 — O — C2H5 is Methoxyethane
The numbering of the parent chain is done so that the carbon atom linked to the -O-atom gets the lowest number.
 SureDen
SureDen