Electrophillic Subsitution of Phenol
Electrophilic substitution of phenol
The -OH group is ortho para directing. it actvates the benzene nucleus towards electrophilic substitution. It directs the incoming group to ortho and para positions in the ring as positions become electron rich due to electronic effect (also mesomeric effect) caused by -OH group.
Common electrophilic aromatic substitution reactions taking place in phenol are as follow:
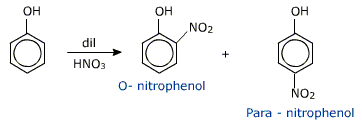
(i) Nitration – phenol reacts with with dilute nitric acid at 293k, phenol yields a mixture of ortho and para nitro phenols.
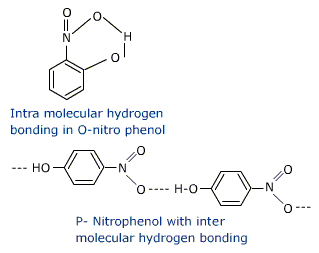
The ortho and para isomers can be separated by steam distillation.since O-Nitro phenol is more steam volatile due to intra molecular hydrogen bonding (chelation ) while p-nitrophenol is less (not )volatile due to intermolecular hydrogen bonding, which causes association of molecules.
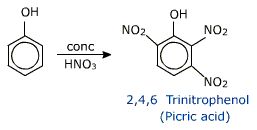
When phenol is treated with conc. HNO3 in presence of conc. H2SO4 , 2,4,6 trinitrophenol.
(picric acid) is formed.
ortho and para nitro phenols are more acidic than phenol
 SureDen
SureDen