Chemical Properties
Chemical properties of monohydric alcohols
They can undergo substitution as well as elimination reaction.
The hydroxyl group present in alcohol is a very reactive group .the reaction of the hydroxyl group consists of either cleavage of C-O Bond or O-H Bond.
ORDER OF REACTIVITY OF CLEAVAGE IN O-H BOND:
primary >secondary >tertiary
ORDER OF REACTIVITY OF CLEAVAGE OF C-O BOND IS:
tertiary >secondary > primary
REACTION OF ALCOHOL ARE CLASSIFIED INTO THREE TYPES:
1) Reactions involving cleavage of -OH bond.
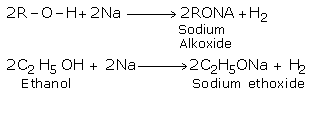
1. # Reaction with ACTIVE metals(ACIDIC NATURE )
Alcohols and react with metals such as sodium, potassium and aluminium to liberate hydrogen and yield corresponding alkoxides and hydrogen.
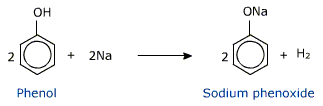
In addition to this, phenols react with aqueous sodium hydroxide to form sodium phenoxides.
ACIDIC NATURE :
ACIDIC CHARACTER OF ALCOHOL AND WATER:
water is a better proton donar than alcohol i.e., alcohols are very weak acids even feeble than water.Alcohol are bronsted acid and alkoxide ion is a better protor acceptor .
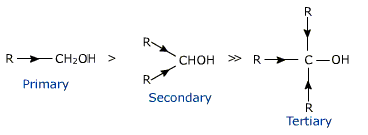
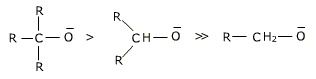
COMPARISON OF ACIDIC CHARACTER OF PRIMARY,SECONDARY AND TERTIARY ALCOHOL:
The acidic character of alcohols is due to polar nature of O-H group.
An electron - releasing alkyl group (-CH3, -C2H5) increases electron density on the oxygen atom tending to decrease the polarity of O-H bond. This decreases the acid strength and so acid strength of alcohol decreases in the following order:
The tertiary alcohols are least acidic as the +IEFFECT of alkyl group would be maximum here while primary alcohols (with only one alkyl group) are most acidic.
The basic strength of the alkoxidesfollow the order:
 SureDen
SureDen