First Law of Thermodynamics
Joule's Law.
Whenever heat is converted into mechanical work or mechanical work is converted into heat, then the ratio of work done to heat produced always remains constant.
i.e. W ∝ Q or W/Q=J
This is Joule’s law and J is called mechanical equivalent of heat.
First Law of Thermodynamics.
It is a statement of conservation of energy in thermodynamical process.
According to it heat given to a system (ΔQ) is equal to the sum of increase in its internal energy (ΔU) and the work done (ΔW) by the system against the surroundings.
ΔQ = ΔU + ΔW
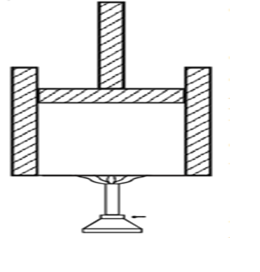
Let us consider a gas inside a cylinder fitted with a movable frictionless piston. The walls of the cylinder are made up of non-conducting material and the bottom is made up of conducting material.
Let the bottom of the cylinder be brought in contact with a hot body like burner. The entire heat energy given to the gas is not converted into work. A part
of the heat energy is used up in increasing its internal energy and the remaining energy is used up in pushing the piston upwards (i.e.) in doing work.
If DQ is the heat energy supplied to the gas, U1 and U2 are initial and final internal energies and DW is the work done by the system, them
of the heat energy is used up in increasing its internal energy and the remaining energy is used up in pushing the piston upwards (i.e.) in doing work.
If DQ is the heat energy supplied to the gas, U1 and U2 are initial and final internal energies and DW is the work done by the system, them
ΔQ = ΔW + (U2 – U1)
ΔQ = ΔW + ΔU
Where ΔU is the change in the internal energy of the system.
Hence, the first law of thermodynamics states that the amount of heat energy supplied to a system is equal to the sum of the change in internal energy of the system and the work done by the system. This law is in accordance with the law of conservation of energy.
Note:
- DQ and DW are the path functions but DU is the point function.
- It makes no distinction between work and heat as according to it the internal energy (and hence temperature) of a system may be increased either by adding heat to it or doing work on it or both.
- Sign conventions
|
ΔQ |
Positive |
When heat is supplied to a system |
|
Negative |
When heat is drawn from the system |
|
|
ΔW |
Positive |
When work done by the gas (expansion) |
|
Negative |
When work done on the gas (compression) |
|
|
ΔU |
Positive |
When temperature increases, internal energy increases |
|
Negative |
When temperature decreases, internal energy decreases |
- Limitation : First law of thermodynamics does not indicate the direction of heat transfer. It does not tell anything about the conditions, under which heat can be transformed into work and also it does not indicate as to why the whole of heat energy cannot be converted into mechanical work continuously.
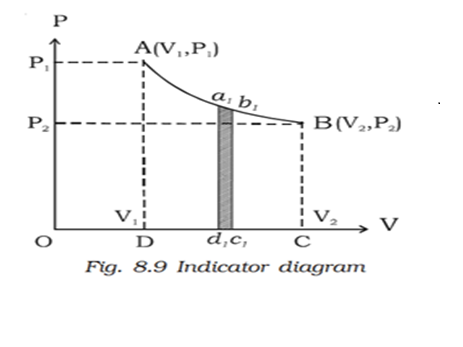
Indicator diagram (P-V diagram)
A curve showing variation of volume of a substance taken along the X-axis and the variation of pressure taken along Y-axis is called an indicator diagram or P-V diagram. The shape of the indicator diagram shall depend on the nature of the thermodynamical process the system undergoes.
Let us consider one mole of an ideal gas enclosed in a cylinder fitted with a perfectly frictionless piston. Let P1, V1 and T be the initial state of the gas. If dV is and infinitesimally small increase in volume of the gas during which the pressure P is assumed to be constant, then small amount of workdone by the gas is dW = PdV
In the indicator diagram dW = area a1b1c1d1
Therefore the total workdone by the gas during expansion from V1 to V2 is
Area ABCD, in the indicator diagram.
Hence, in an indicator diagram the area under the curve represents the work done.
 SureDen
SureDen