Cyclic and Non-cyclic Process
Cyclic and Non-cyclic Process in Thermodynamics
Cyclic Process: A cyclic process consists of a series of changes which return the system back to its initial state.
Non Cyclic Process: In non-cyclic process the series of changes involved do not return the system back to its initial state.
(1) In case of cyclic process as Uf = Ui
Therefore ΔU=Uf-Ui=0 i.e. change in internal energy for cyclic process is zero and also ΔU ∝ ΔT
Therefore ΔT = 0 i.e. temperature of system remains constant.
(2) From first law of thermodynamics ΔQ=ΔU+ΔW
ΔQ=ΔW i.e. heat supplied is equal to the work done by the system. [As ΔU = 0]
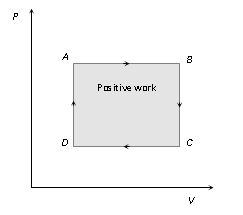
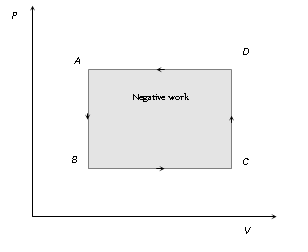
(3) For cyclic process P-V graph is a closed curve and area enclosed by the closed path represents the work done.
If the cycle is clockwise work done is positive and if the cycle is anticlockwise work done is negative.
(4) Work done in non cyclic process depends upon the path chosen or the series of changes involved and can be calculated by the area covered between the curve and volume axis on PV diagram.
 SureDen
SureDen