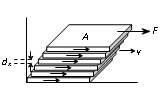
Viscosity and Newtons law of Viscous Force
In case of steady flow of a fluid when a layer of fluid slips or tends to slip on adjacent layers in contact, the two layer exert tangential force on each other which tries to destroy the relative motion between them. The property of a fluid due to which it opposes the relative motion between its different layers is called viscosity (or fluid friction or internal friction) and the force between the layers opposing the relative motion is called viscous force.
Consider the two layers CD and MN of the liquid at distances x and x + dx from the fixed surface AB, having the velocities v and v + dv respectively. Then
denotes the rate of change of velocity with distance and is known as velocity gradient.
According to Newton's hypothesis, the tangential force F acting on a plane parallel layer is proportional to the area of the plane A and the velocity gradient
in a direction normal to the layer, i.e.,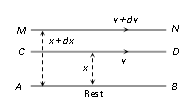
Where h is a constant called the coefficient of viscosity. Negative sign is employed because viscous force acts in a direction opposite to the flow of liquid.
Hence the coefficient of viscosity is defined as the viscous force acting per unit area between two layers moving with unit velocity gradient.
(1) Units : dyne-s-cm–2 or Poise (C.G.S. system); Newton-s-m–2 or Poiseuille or decapoise (S.I. system)
1 Poiseuille = 1 decapoise = 10 Poise
(2) Dimension : [ML–1T–1]
(3) Viscosity of liquid is much greater (about 100 times more) than that of gases i.e
Example : Viscosity of water = 0.01 Poise while of air = 200 m Poise
(4) With increase in pressure, the viscosity of liquids (except water) increases while that of gases is practically independent of pressure. The viscosity of water decreases with increase in pressure.
(5) Difference between viscosity and solid friction : Viscosity differs from the solid friction in the respect that the viscous force acting between two layers of the liquid depends upon the area of the layers, the relative velocity of two layers and distance between two layers, but the friction between two solid surfaces is independent of the area of surfaces in contact and the relative velocity between them.
(6) From kinetic theory point of view viscosity represents transport of momentum, while diffusion and conduction represents transport of mass and energy respectively.
(7) The viscosity of thick liquids like honey, glycerin, coaltar etc. is more than that of thin liquids like water.
(8) The cause of viscosity in liquids is cohesive forces among molecules where as in gases it is due to diffusion.
(9) The viscosity of gases increases with increase of temperature, because on increasing temperature the rate of diffusion increases.
(10) The viscosity of liquid decreases with increase of temperature, because the cohesive force between the liquid molecules decreases with increase of temperature
Relation between coefficient of viscosity and temperature; Andrade formula
Where T= Absolute temperature of liquid, r = density of liquid, A and C are constants.
 SureDen
SureDen