Shape of Drops
Whether the liquid will be in equilibrium in the form of a drop or it will spread out; depends on the relative strength of the force due to surface tension at the three interfaces.
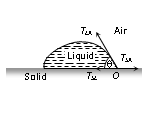
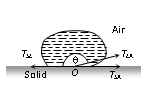
TLA = surface tension at liquid-air interface, TSA = surface tension at solid-air interface.
TSL = surface tension at solid-liquid interface, q = angle of contact between the liquid and solid.
For the equilibrium of molecule
TSL + TLA cosq = TSA or
Special Cases
|
TSA > TSL, cosq is positive i.e. . This condition is fulfilled when the molecules of liquid are strongly attracted to that of solid. Example : (i) Water on glass. (ii) Kerosene oil on any surface. |
|
TSA < TSL, cosq is negative i.e. . This condition is fulfilled when the molecules of the liquid are strongly attracted to themselves and relatively weakly to that of solid. Example : (i) Mercury on glass surface. (ii) Water on lotus leaf (or a waxy or oily surface) |
|
(TSL + TLAcosq) > TSA In this condition, the molecule of liquid will not be in equilibrium and experience a net force at the interface. As a result, the liquid spreads. Example : (i) Water on a clean glass plate. |
 SureDen
SureDen