1st Law of Thermodynamics
Internal energy → The total amount of energy stored in a substance is known as Internal energy. Total internal energy is the sum of the various types of small amount of energy i.e. kinetic energy, rotational energy, vibrational energy, nuclear energy or any other type of energy associated with the substance. So
Internal energy
E = EkI + Ev + Et + En
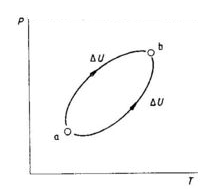
State Function → A thermodynamics quantity, the change in the value of which, depend upon the initial & final state of the system & is independent of the path by which change has been brought about is called a state function.
Path function → A thermodynamic quantity, the change in the value of which, depend on the path followed by the system is known as path function.
Heat & work are path function. While internal energy is a state function
In both the cases, energy changes is same hence, internal energy is a state function.
1st law of Thermodynamics
- It state that energy can neither be created nor be destroyed. It can change from one form to another form.
- It is impossible to construct a machine which can produce work without consuming energy.
Mathematical Expression for 1st law
E2 = E1 + q + w
E2 – E1 = q + w
ΔE = q + w ← If work is done on the system
If work is done by the system ΔE = q
 SureDen
SureDen