Schrodinger Equation
To give sense to the probability approach, Schrodinger in1926 derived an equation known after his name as Schrodinger's wave equation. This equation is the basic of wave mechanics and is based upon the idea of the electron as standing wave around the nucleus. If the electron has wave like nature, it should obey the same equation of motion as all other known type of wave. On the basis of simple idea Schrodinger derived an equation which describes the wave motion of an electron wave along of the three axis x, y, z called Schrodinger wave equation.
Schrodinger's wave equation correlates the wave property of the electron with its energy. During the derivation of this equation, he took the following under consideration.
1.De-Broglie's wave particle duality equation
2. Heisenberg's uncertainty principle
3. Bohr's concept of quantized energy levels.
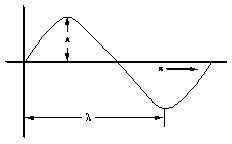
The equation for the standing wave comparable with that of a stretched string is
Ψ= A sin(2πx/λ)………………..(i)
Where Ψ (sigh) = amplitude at displacement x and is wave function
A = constant
x = displacement direction
λ = wavelength.
Differentiating equation (i) twice with respect to x we get,
As total energy (E) = Kinetic Energy + Potential Energy
Or E = (1/2)mv2 + U [P.E. = U]
Again
According to de-Broglie's equation
λ =h/mv
Putting the value of v2 from eq (iii) on eq (iv) we get,
Putting the value of eq (v) on eq (ii) we get
Equation (vi) is a Schrodinger wave equation for the wave motion in one dimension only i.e. x-axis. For electron moving in a three dimension space it is modified as:
Where (del-square)[Laplacian operator]=
Equation (vii) and (viii) are Schrodinger wave equation expressions.
The valid values of are called Eigen functions, and the values of E corresponding to this Eigen functions are called Eigen values. The Eigen values are found to be more or less the same energy values given by Bohr's in different orbit.
For Ñ° to be valid it should satisfy following conditions:
i. Ñ° must be finite and continuous
ii. It should be single valued
iii.
must be continuous functions of x, y, and z coordinates respectively.
Ψ represent the three dimensional amplitude of electron wave at various points surrounding the nucleus, and is called orbital. However, Ψ 2, gives the probability of finding electron of certain energy at a space inside the atom.
The value of Ψ may be real or imaginary. If Ψ is real, Ψ 2 also is real. Thus Ψ 2 gives the probability of finding electron.
But if Ψ is imaginary Ψ Ψ * gives the probability of finding electron wave
let Ψ = a + ib (imaginary quantity)
Ψ Ψ * = (a+ib) (a–ib) = a2+b2 (real)
Note: The probability should always be real and positive.
 SureDen
SureDen