Rutherford model
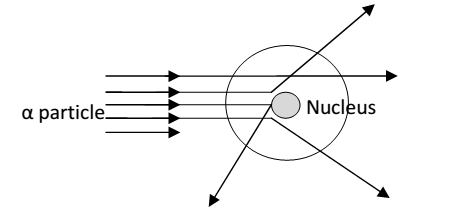
Rutherford model of atom- IN 1911 Rutherford his coworkers performed a series of experiment known as scattering experiments. He bombarded X particles or thin gold foil (i.e. 100 mm thick). He observed following results.
- Most of the X-particles (99.9%) pan through there + produce bright spot on screen.
- Few particles were deflected at different angles.
- Very few particles (one in 20000) were deflected from the above observation, Rutherford made following conclusions.
- Since most of X-particle could pan through them it shows that most of the pace in an atom is hollow.
- Few particles were deflected from their path it must be concluded that inside an atom a heavy positive charged mass present in 94 atom. Moreover this mass must be occupied in very small space in atom
-
Bouncing back of X-particle from the foil indicate that the direct collision with the heavy positive charge mass, which is extremely small as compared to total volume of atom.
Features of Rutherford model
- Atom consist of 2 parts (a)Nucleus (b)extra nuclear part.
Nucleus is present in centre + space around the nucleus called extra nuclear part.
- Two size of nucleus is very small as compared to extra nucleus part(10-10m)
- The entire mass of an atom is due to nucleus which contains Proton and Neutrons while electrons present in extra nucleus part with negligible mass.
- Electrons in extra nuclear portion are not stationary and are revolving around the nucleus in a circular orbits. They are also called planetary electrons.
- The force of attraction of electron with the nucleus is balanced centrifugal force acting away from the nucleus thus electron do not leave their orbits.
Atomic no = no. of proton in nucleus
(proton no) = no. of electron in natural atom
Mass no = no. of proton + no. of neutrons.
Nucleus – proton + neutrons present in nucleus no mass no nearly equal to atomic mass
Mass – not a whole number
Mass number – always whole number
Isotopes –atoms of same element having same at no. but different mass no. are called Isotopes
11H, 21H, 31H (protium, deuterium & tritium )
Isobar- atoms of different elements possess same mass no.
4018Ar, 4019K, 4020Ca (differ in no. of P+N )
Isotones- atoms of different elements which contain the same no. of neutrons called isotones. (differ in
at no. + mass no.)
146C, 157N, 168O (each of then contains 8 neutrons )
Isoelectronics – atoms or Ions containing same no. of electrons are called Isoelectronics.
N---, O--, F-, Na+, Mg++,Ai+++,Ne Each contains 10 electrons.
 SureDen
SureDen