Radioactivity
Radioactivity – discovered by Henry Becquerel.
“ spontaneous emission of active radiation from a radioactive substance called radioactivity .Those elements emitting such radiations called radioactive elements.
3 types of radiations are emitted: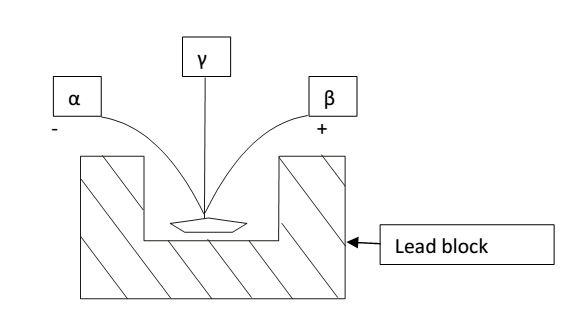
- α-rays – 1. Deflected towards negative plate
2. having positive charge called X-rays
The particle present in them called X-particle. Each X-particle has chage+2 unit +mass unit (He24)
- β- rays- 1. Deflected towards positive plate
2. Having negative charge called β-rays
The particle present in them called β-particle.
β-particle possess same charge + mass as electron (-1 oe)
- γ-rays- 1.They remains un-deflected
2.They are simple electromagnetic radioactive (ϒoo)
Related Keywords
 SureDen
SureDen