Plancks Quantum Theory
Particle nature of electromagnetic radiation
(Planck’s quantum theory)
- Radiant energy is emitted or absorbed not continuously but discontinuously in the form of small packet of energy. Each packet of energy called Quantum.
- Energy of each quantum is directly proportional to the frequency of radiation
E = hν h= Planck’s constant (6.62 x 10-27 every sec.)
- Total amount of energy emitted or absorbed will be some whole number of quanta
E = nh
- In case of light, quantum of energy is called photon
One Einstein of energy
Energy possess by 1 mole of quanta = No h (c/λ)
No = Avogadro number
Photoelectric Effect – When radiation with certain min. frequency (νo) fall on the surface of a metal, the electrons are ejected from the surface of metal. This phenomenon is called photoelectric effect + such electron are emitted are called photoelectrons.
For a particular metal, thereafter exist a threshold frequency (νo)
If frequency is less then threshold frequency no electrons are ejected.
Threshold frequency – (νo) i.e. min. frequency required to eject electron from the metal surface called threshold frequency
Work function –
- (νo) minimum energy required to eject electron called work function.
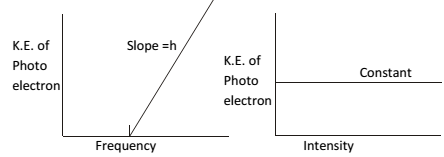
- KE of ejected electron depend upon the frequency of incident radiation but independent to its intensity.
- The number of photoelectron ejected is proportional to the intensity of incident radiation.
Above observations can’t explained by electromagnetic wave theory because radiations are cosistisous so, radiations of all frequencies should be able to eject electrons. Thus this theory explain that energy of ejected electron should depend upon the intensity of incident radiations.
Explanation of Photoelectric Effect on particle nature
- When light falls on metal surface photon gives entire energy to metal electron. If energy of photon is sufficient, electron ejected from metal surface such energy called threshold frequency (νo)
- If frequency of incident light (ν) is more than threshold frequency (νo), then excess energy (hν -hνo) is imparted to the electron as KE.
KE of ejected electron. ( ½mυ2) = hν - hνo KE = h (ν - νo)
Or hν = hνo + ½mυ2 or hν = hνo +KE
Or hν = hνo + ½mυ2
Hence greater the frequency of incident light, greater is the kinetic energy of emitted e-
-
By increasing the intensity of light of a given frequency number of photons increases, but does not increase the energies of photons. Hence by increasing the intensity of light more electrons are ejected.
Electron volt – Energy acquired by an electron when it is accelerated through a potential difference of one volt called electron volt
1 e V = charge of one electron x 1 volt
= 1.602 x 10-19 c x 1 v = 1.602 x 10-19 J
 SureDen
SureDen