Evidences and Limitations
Evidences in Favors of Bohr model
- Stability of an atom – According to Bohr electron revolving around the nucleus in a particular orbit and can’t loss energy. Hence electron loss energy only when they jump from higher to lower level. This is only possible when electron absorb energy from external source. Therefore electron revolve in a circular fixed energy level and do not loss energy hence atom is quite stable.
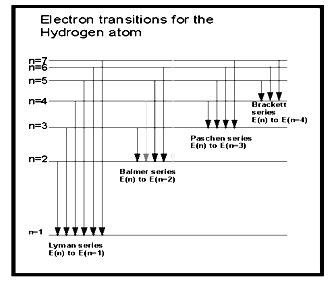
- Atomic spectra of hydrogen atom- When an electric discharge is passed through the hydrogen gas at a reduced pressure, contained in a tube due to bombardment of hydrogen molecules by electrons hydrogen atoms are produced. Some hydrogen atoms get existed due to absorption of energy. When atoms returns to their original state, energy is emitted in the form of photons of light of definite wavelength or frequency.
Hydrogen possess only one electron but its atomic spectrum consists of a large number of lines because hydrogen gas contains a large no. of molecules, when gas is heated at high temper in electric discharge is passed, the hydrogen molecules splits into hydrogen atoms. The electrons in different hydrogen atom absorb different energy levels. When they returns to lower energy level or ground state in one or more jumps thus they emit different amount of energies and produced a large number of lines in atomic spectra of hydrogen.
The wave no. of each line of spectrum is given by
R= Rydberg constant (109677 cm-1)
n2>n1 called orbit number
For Lyman series n1 =1 n2=2,3,4,5,6….comes in U.V.
For Balmer series n1 =2 n2=3,4,5,6….comes in visible
For Paschen series n1 =3 n2=3,4,5,6,7….comes in infra red
For Bracket series n1 =4 n2=5,6,7,8….comes in infra red
For Pfund series n1 =5 n2=6,7,8,9….comes in infra red
By substituting the value n1 and n2 , the frequency of any radiation can be calculated.
The spectrum of other atom are complex consisting of very large no. of lines, whose wave length can’t be related with Rydberg equation.
- Calculate the energy of the Electron :-
Bohr calculate the energy of an electron moving in a particular orbit of hydrogen atom.
The energy of an electron in vth orbit has been found to be
By substituting the value of m,e,h the expression become
If n=1 we get energy of an electron = - 1312 KJ
n=2 we get energy of an electron = - 327.9 KJ
n=3 we get energy of an electron = - 145.7 KJ
1ev = 1.602 x 10-19 J
Limitations of Bohr model
- Bohr model of atom successfully explaining atomic spectra of hydrogen atom and hydrogen like particles containing single electron. (He+, Li++) but it fails to explain the atomic spectra of atoms containing many electrons.
- According to Bohr electron move in circular orbits around the nucleus i.e. Planer motion. But actually this motion is three dimensionally.
- It fail to explain the shape of molecules.
- This theory fails to explain the splitting of spectral lines under the influence of magnetic field ( called as Zeeman effect) and electric field (called as Stark effect)
:- under the influence of strong magnetic field, each of the two yellow lines of sodium spectrum is split up into two five lines . Bohr could not explain this fact.
- It could not explain dual nature of electron
De Broglie principle- wave particle duality of matter called as de Broglie principle.
Wave particle duality of matter called as de Broglie principle.
h= Planck’s constant
v= velocity.
 SureDen
SureDen