Dual Nature of Matter
Dual nature of matter – radiation-
The wave associated with a particle is called matter wave or de Broglie wave.
Derivation – in case photon, if it is assumed to have wave character, its energy is
E = hv or h (c/λ) according to Planck’s equation theory ------------ (1)
If photon is suppose to wave particle nature
Its energy is E = mc2 (according to Einstein equation) ------ (2)
From equation (1) and (2) hv = mc2
λ = h/mc h = Planck’s constant
Such equation applicable for any material particle.
For any material particle (like e)
λ = h/mv or λ = h/p
Such equation called de Broglie equation.
Difference Between particle in space i.e.
|
Particle |
Wave |
|
|
|
|
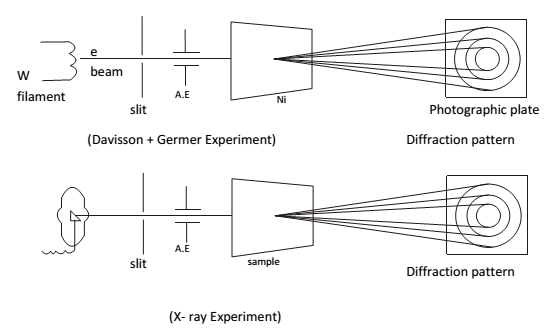
Experimental verification of wave nature of electrons.
Wave nature of e- verified division and garner. They produce a beam of light (e) from W filament and pan over electric field so as to accelerate their speed. When there electron fall on Ni or gold a diffraction pattern (dark bright concentric rings) were imprinted on photographic plate. Such diffraction pattern of electron was similar to X-rays possess wave character, so electron calculated by diffraction experiment same as de Broglie’s.
Particle nature of electron- When an electron strikes on Zns screen. It produce a spot of light called scintillation point. Thus striking electron must be localized and not spread not like a wave. Thus electron behave like a particle.
Significance of de Broglie equation
- It is applicable for microscopic particles (like electron, proton) because wavelength can be easily measured and Number significance in every day like because we observe macroscopic particles.
- Electron microscope based on de Broglie principle and measure the wavelength of very small object.
Difference between electromagnetic wave and matter wave
|
Electromagnetic waves |
Matter waves |
|
(1)They are associated with electric and magnetic field, perpendicular to each other and to the direction of propagation. |
(1)They are not associated with E. F and M.F. |
|
(2)They do not require any medium for propagation(i.e. pass in vacuum) |
(2)They require any medium for propagation(i.e. not pass in vacuum) |
|
(3)They travel with the same speed of light |
(3)The travel with lower speed. |
|
(4)They leave the source |
(4)They do not leave the moving particle |
|
(5) λ = e/v or c = vλ or λv = e/ λ |
(5) λ = h/mv |
Derivation of Bohr postulates of Quantization of angular momentum from de Broglie equation.
According to Bohr model, electron revolves around the Nucleus in a circular orbit and only those secular orbits ore permitted in which angular momentum of the electron is integral multiple.
Of h/2π i.e. mvr = nh/2π
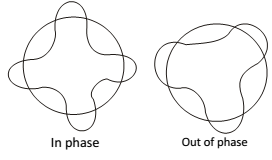
But according to de Broglie concept, the electronics not only a particle but has a wave. A wave to be completely in phase, if the circumstance of the orbit must be equal to integral multiple of wavelength (λ)
2πr = n λ but λ = h/mv
2πr = nh/mv or mvr = nh/2π
This is Bohr postulate of angular momentum.
Calculation of de Broglie wavelength of the electron from the potential applied to accelerate
If accelerating potential (V) applied on electron beam, the energy acquired by an electron is expressed in electron volt (ev)
ev = charge on electron (c) x potential
λ = 12.26/√V
 SureDen
SureDen