Measurement of Mass, Volume, Pressure and Temperature
(1) Mass of gas is measured by determining the wt. of empty container and then container filled wet gas and then taking their difference which will give mass of gas.
No of molar = masses gram/molar mass
(2) The volume of gas is equal to volume of container is which gas is present. 25’ SI unit is m3.
Other unit are 1 m3 = 103 dm3 = 106 cm3
1 ml = 1 cm3
1 litre = 103 cm3 = 1 dm3 = 10-3 m3
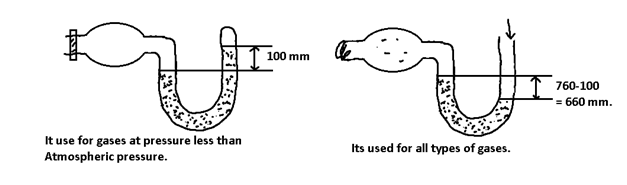
(3) Pressure:- The atmospheric pressure is measured by barometer. The height of Hg – column above the level of Hg is dish is measure of atmospheric pressure at that place.
The pressure of gas is measured by manometer.
Manometer is of 2 types:-
Pressure = Force/Area = ((A x h x ρ) x g/A)
Where A → Area of cross section
h → height to which Hg – rises
ρ → density of Hg g → acceleration due to gravely
(A x h x ρ) = mass of mercury so P = ρ h g
Standard or Normal atmospheric pressure is
1 atm = 76 cm = 760 mm or 760 torr
Now unit of P uses is ‘bar’
1 bar = 0.987 atm
1 atm = 101325 Pa or non-2 or kg m-1s-2
1 bar = 105 Pa
Temperature is measured on 3 difference scales
(i) Centigrade or Celsius scale (ii) Fahrenheit scale
(iii) Kelvin scale
0°C = 273.15 k
Relation between – °C & F
°C = 5/9 (°F – 32)
 SureDen
SureDen