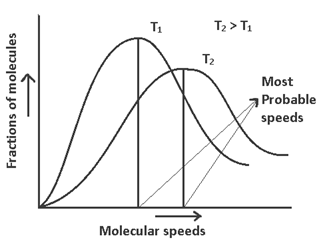
Maxwells Distribution of Molecular speeds
Maxwell’s distribution of molecular speeds/energies
(1) The fraction of molecules possessing very low & very high speeds are very small
(2) The speed which is possessed by very large no. of molecules is called most probable speed.
(3) On increasing temperature the graph is shifted towards right hand side & (Vmp) most probable velocity increases & fraction of molecules possessing (Vmp) also increases
Kinetic Gas equation → Its PV = 1/3 mnu2
If n → 1 mole then
m x n = M (molar mass)
So PV = 1/3 Mu2
P → Pressure exerted by
Where m → Mass of each molecular of gas
n → n° of molecules present is volume V
u → Root mean square velocity
Types of speeds → (i) Most probable speed (vmp)
vmp = (2RT/M)1/2
(ii) Root mean square velocity (vrms) = (v12 + v22 + ….vn2/n)1/2
Also vrms = (3RT/M)1/2
(iii) Average velocity (vav) v = (v1 + v2 + v3……../n)
Also vavg = (8RT/πM)
Relation bet there
Vmp : vav : vrms
(2RT/M)1/2 : (8RT/ πM)1/2 : (3RT/M)1/2
 SureDen
SureDen