Gas Laws 1
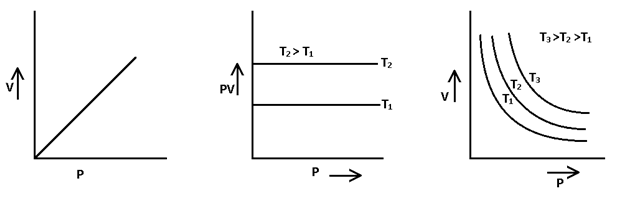
(i) Boyle’s Law → “The volume of given mass of a gas is inversely proportional to its presence at constant temperature.”
Mathematically V ∝ (1/P) or V = K(1/P) or PV = K where K → constant .
If P1 V1 are initial P and V & P2, V2 are final P & V
Then
P1 V1 = P2 V2 at const. temperature
Graphically,
P – V curve at constant temperature is known as Isotherm.
Significance → At altitudes, atmospheric P is low, so air is less dense. So less oxygen is available for breathing & person feels uneasiness, headache etc. & this is called Altitude sickness that’s why mountaineers have to carry oxygen cylinders with them.
Numerical:- A balloon filled with an ideal gas is taken from surface of sea, deep into depth of 100m. What will be its new volume in terms of its original volume?
Solution:- P at surface = 76 cm of Hg = 76 x 13.6 cm of H2O = 10300 cm = 10.3
P at 100 m depth = 100 + 10.3 = 110.3 m
Applying at surface at 100 m depth P1V1 = P2V2
10.3 x V1 = 110.3 x V2 or V2 = (10.3/110.3) x V1 = 0.093 V = 9.3% of V
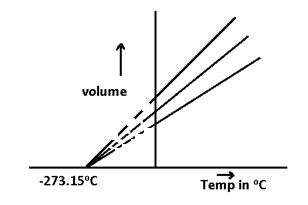
When V – t graph for gases was extrapolated then all the lines meet at -273.15°C which indicates that the volume of all gases become zero at -273.15°C As this is the min. possible temperature therefore below this temperature the volume of gases will become negative which is not possible so,
Absolute zero = 0K = -273.15°C
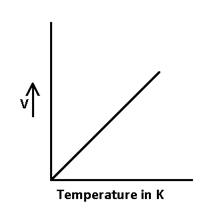
The advantage of Kelvin scale is that, volume of gas is diversity proportional to temperature in Kelvin.
So other definition is “the volume of given mass of gas is directly proportional to its temperature in degree Kelvin at const. pressure.”
So V ∝ T
V1/T1 = V2/T2
Or (V/T) = constant at const. Pressure.
Significance of Charle’s law → Hot air is lighter than atmospheric air. So hot air is filled in balloons used for meteorological observations.
GAY-LUSSAC LAW/AMOTON’S LAW → It states that “the pressure of a given mass of gas increases or decreases by (1/273) of its pressure at 0°C for every 1°C size or fall in temperature.
Mathematically
Pt = P0 + P0 x (1/273) x t = P0(1 + t/273)
P0 & Pt are P of gas at 0°C & t°C reply.
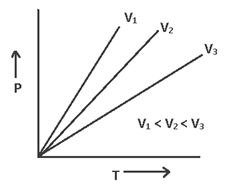
Also Pt ∝ T or P ∝ T or (P/T) = const. or
P1/T1 = P2/T2 at const. V. “the volume remaining const. the pressure of given mass of gas is directly proportional to its temperature.”
AVOGADRO’S LAW → It states that “equal volume of all the gases under similar conditions of temperature and pressure contain equal number of molecules
At STP i.e. 0°C & 1atm. Pressure. Molar Volume = 22.4 L mol-1 at 0°C & 1 bar Pressure, Molar Volume = 22.71 L mol-1
At standard ambient temperature and pressure (SATP) i.e. 298.15 K and 1 bar pressure Vm = 24.78 L mol-1
So V ∝ n n → n of moles also n = w/m
V = K (m/M) or M = K (m/V) (m/V = density (d))
So density of gas is directly proportional to its Molecular Mass
Question:- On a ship sailing is pacific ocean as in 23.4°. A balloon is filled with 2L air to the volume balloon when the slip reach ocean where temperature is 26.1°C
Solution:- (n/T1) = (V2/T1) or (2 L/296.4 K) = (V2/299.1 K) or V2
 SureDen
SureDen