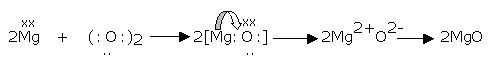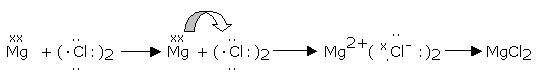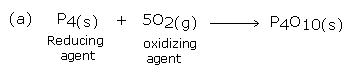Electronic interpretation of oxidation and reduction
Electronic interpretation of oxidation and reduction
From the above reactions, it can be seen that the oxidation-reduction reactions involve transfer of electrons from one species to another. Thus, the oxidation-reduction reactions may also be defined as follows.
1) Oxidation: Oxidation is a chemical process in which there is a loss of electrons.
Or
(Oxidation is an increase in the oxidation number of elements in given substance). The species, which gets oxidized becomes more positive or less negative.
Na → Na+ + e- (Na is oxidized to Na+)
Fe2+ → Fe3+ + e- (Fe2+ is oxidized to Fe3+)
2) Reduction: Reduction is a chemical process in which there is a gain of electrons. The species, which gets reduced becomes more negative or less positive.
OR
(Reduction is an decrease in the oxidation number of elements in given substance).
Cl2 + 2e- → 2Cl- (Cl2 is reduced to Cl-)
S + 2e- → S2- (S is reduced to S2-)
3) Oxidizing agent:
Oxidizing agent is the species, which accepts electrons.
Or
(That increases the oxidation number of an element).
Sn4+ + 2e- → Sn2+ (Sn4+ is an oxidizing agent)
F2 + 2e- → 2F- (F2 is an oxidizing agent)
4) Reducing agent:
Reducing agent is the substance, which loses electrons.
Or
(That lowers the oxidation number of an element)
Hg+ → Hg2+ + e- (Hg+ is a reducing agent)
Fe2+ → Fe3+ + e- (Fe2+ is a reducing agent)
5) Reducing as well as oxidizing agent:
A substance can act as reducing as well as oxidizing agent if oxidation number of one of its element is between the maximum and minimum value. e.g,
In HNO3 Oxidation number of N = 3 which is intermediate of +5 and 0.
6) Oxidation number and acid strength:
Greater the oxidation number of elements in oxyacids, the greater is the acid strength.
+7 +5 +3 +1
HCIO4 > HCIO3 > HCIO2 > HCIO
7) Oxidation and Reduction (Electron Transfer Process)
Magnesium when burnt in air gives magnesium oxide in accordance with the reaction,
2Mg(s) + O2(g) → 2MgO(s)
Here, magnesium combines with oxygen to form magnesium oxide, i.e., oxygen is added to magnesium. According to the classical concept of oxidation and reduction, the addition of oxygen to magnesium is termed as the oxidation of magnesium.
The formation of magnesium oxide on the basis of the electronic configurations of magnesium and oxygen can be explained as follows:
Magnesium oxide (MgO) consists of Mg2+ and O2-.
This means that during the formation of MgO, Mg is converted into Mg2+ and O into O2-. This is possible only when a magnesium atom (Mg) loses two electrons to form Mg2+ and an atom of oxygen (O) gains two electrons to form oxide ion (O2-). The following reactions should occur.
Mg → Mg2+ + 2e-
O + 2e- → O2-
Or O2 + 4e- → 2O2-
In the overall reaction between magnesium and oxygen as electron does not appear either on the reactant-side nor on the product-side, the number of electrons lost by magnesium must be equal to the number of electrons gained by oxygen. In this way the number of electrons are balanced. To balance electrons, the first reaction is multiplied
2Mg → 2Mg2+ + 4e-
Then, adding the other two equations one obtains,
Thus, two electrons are transferred from a magnesium atom to an oxygen atom in the reaction between magnesium and oxygen. This electron-transfer process is possible only when one of the species is capable of losing while the other is capable of gaining an electron, both being present in the reaction. The loss and the gain of electrons occurs simultaneously.
The process involving loss of electrons is termed as oxidation, while that involving gaining of electrons is termed reduction. Therefore, oxidation and reduction reactions involve electron-transfer from one reactant to another.
Such reactions in which electrons are transferred from one reactant to another are called "redox reactions" involving both oxidation and reduction.
For example,
Problem
1. In the following reaction, label the oxidizing agent and the reducing agent.
(a) P4(s) + 5O2(g) → P4O10
(b) CO(s) + Cl2(g) → COCl2(g)
Solution
(b) CO(g) + Cl2(g) → COCl2(g)
 SureDen
SureDen