Water
Water:
Structure of water:
- In the Earth's biosphere, water is essential to all forms of lifeAbout 75% of the Earth's surface consists of water.
- Water is present as either Solid, Liquid or vapour. All basic and metabolic functions of our body are dependent upon water.
- All species on this planet need water as basic need. Most fresh water is not readily available, being under the ground, or in the form of glaciers and frozen lakes.
- The fraction of water available for human civilization is estimated at less than 0.003% of the total global water availability.
- Aquatic marine life in oceans, seas and other saline inland water bodies, enjoy 97.3% of the total global supply of water supply.
Structure of water molecule in the gas phase:
A molecule of water consists of two hydrogen atoms joined to an oxygen atom by covalent bonds.
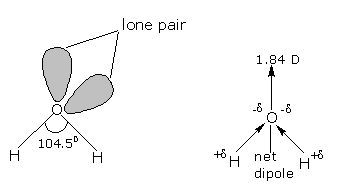
The oxygen atom has six electrons (1s2 2s2 2p4) in its outermost shell. The 's' and 'p' orbitals of the valence shell are sp3 hybridized to form four sp3hybrid orbitals oriented tetrahedrally around the oxygen atom. Two of the hybrid oribtals are singly occupied, while the lone pairs of electrons occupy the other two. Each singly occupied sp3 orbital overlaps with the half-filled 1s orbital of 'H' atom. Thus, oxygen is bonded to the two hydrogen atoms by two O-H covalent bonds, and there are two lone-pairs of electrons on the oxygen atom. Due to the presence of two lone-pairs of electrons on the O atom, the H-O-H bond angle is 104.5°, which is slightly less than the tetrahedral angle of 109°28'. Therefore, the structure of water molecule is an angular or bent structure.
Polarity of water molecule:
Oxygen is more electronegative than hydrogen. Its high electronegativity causes the oxygen atom to pull the shared pairs of electrons more towards itself. As a result, the O-H bond acquires polarity. Since the two O-H bonds in water are inclined at an angle, hence the net dipole moment of water molecule is not zero. The actual dipole moment of water molecule is 1.84 debye or D (the unit of dipole moment)
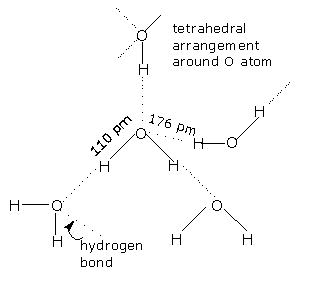
Structure of water molecule in the liquid state:
In liquid state, water molecules are held together by intermolecular hydrogen bonds. Each oxygen atom can form two hydrogen bonds utilizing both the lone pairs on it. And most of the theories states that, water in liquid state exists in the form of various water molecules joined together by hydrogen bonds. These continually forming, collapsing and reforming
Structure of water in solid state:
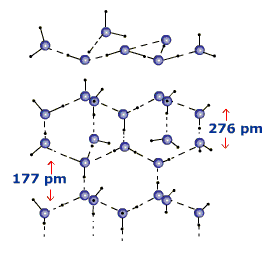
Depending on the conditions for freezing of water, the solid form of water i.e., ice, can exist in different crystalline forms. In 'normal' hexagonal ice, four other oxygen atoms tetrahedrally surround each oxygen atom. One hydrogen atom lies in between each pair of oxygen atoms. Thus, each and every hydrogen atom is covalently bonded to a oxygen atom and linked to another oxygen atom by a hydrogen bond.
When ice melts some of the hydrogen bonds are broken and the water molecules become more closely packed. It results in an increase in the density of water above its melting points
Hexagonal ice
 SureDen
SureDen