Properties of Hydrogen as Alkali Metals
Properties of Hydrogen that Resemble Alkali Metals:
Electronic configuration
Hydrogen atom has one electron in its valence shell like alkali metals.
Valency
Hydrogen generally shows + 1 valency like alkali metals.
Metallic character
It readily loses its electron to form a positive ion like other alkali metals.
H → H+ + 1 e-
Na → Na+ + 1 e-
Combination with non-metals
Hydrogen, like the alkali metals, combines readily with non-metals like halogens, oxygen, sulphur, etc.
Reducing agent
Hydrogen is a good reducing agent like other alkali metals.
Properties of Hydrogen that Resemble Halogens
Non-metallic character
Hydrogen is a non-metal like halogens.
Atomicity
Like halogens, hydrogen is diatomic (H2) whereas metals are monoatomic.
Valency
Like halogens, hydrogen may also show 1 valency by accepting an electron. Example: NaH (Sodium hydride)
Nature of compounds
Hydrogen combines with non-metals like carbon, silicon, etc. to from covalent compound like halogens.
Examples: CH4 and CCl4.
Atomic hydrogen, Nascent hydrogen
Atomic Hydrogen
Langmuir, in 1915, obtained atomic hydrogen by dissociating on a hot filament of tungsten or platinum. The dissociation of molecular hydrogen is an endothermic process.
H2(g) + 436KJ → H + H
The atomic hydrogen is stable only for a fraction of a second and immediately converts back to its molecular form, liberating a large amount of energy.
H + H → H2 + 436KJ
Atomic hydrogen is extremely reactive, being more reactive than ordinary, nascent, or adsorbed oxygen. When it is passed over metals or non-metals, it forms hydrides at normal temperatures, excepting for nitrogen, to which it does not react.
Atomic hydrogen is an extremely powerful reducing agent, reducing oxides chlorides and sulphides of some metals like Ag, Hg, Cu, etc. to metals at ordinary temperature.
Nascent Hydrogen
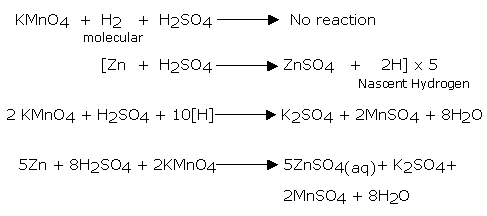
The hydrogen gas, which is just liberated as a result of a chemical is called nascent hydrogen. It is obtained by passing di-hydrogen gas through an electric arc between two tungsten rods. The electric arc maintains a temperature around 4000°C. As the molecules of di-hydrogen gas pass through the electric arc, these absorb energy and turns into atoms as nascent hydrogen. It is more reactive than ordinary hydrogen and pink in color.
The following reaction process is shown by nascent oxygen, but not by ordinary oxygen: If ordinary hydrogen is passed through acidified KMnO4 it does not get de-colorized. However, if zinc pieces are added to the same solution, bubbles of hydrogen rise up through the solution and the color is discharged due to the reduction of KMnO4 by nascent hydrogen.
 SureDen
SureDen