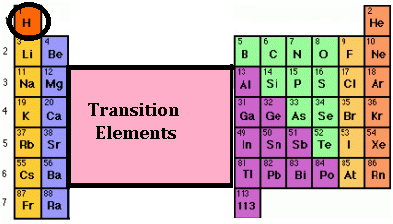
Position of hydrogen in periodic table
Position of hydrogen in periodic table:
Hydrogen is the first element of the periodic table. Its atomic number is 1, which indicates the presence of only one electron in the atom of hydrogen. This electron is present in its first shell. Hydrogen resembles both alkali metals (group I A) as well as halogens (group VII A) therefore its position is said to be anomalous.
Some Basic Features of Hydrogen
|
Symbol |
H |
|
Molecular formula |
H2 |
|
Atomic number |
1 |
|
Mass number |
1 |
|
Atomic mass |
1.008 |
|
Molecular mass |
2 |
|
Electronic configuration |
1 |
|
Valency |
+ 1 or -1 |
|
Isotopes of Hydrogen |
11H Protium |
|
21H Deuterium |
|
|
31H Tritium
|
Related Keywords
 SureDen
SureDen