Ortho and Para hydrogen
Ortho and Para hydrogen:
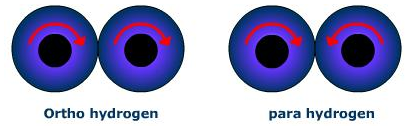
A molecule of dihydrogen contains two atoms, in which the nuclei of both the atoms are spinning. Depending upon the direction of the spin of the nuclei, the hydrogen molecules are of two types:
Ortho hydrogen molecule: In which the spins of both the nuclei are in the same direction.
Para hydrogen molecules: In which the spins of both the nuclei are in the opposite direction.
Ordinary di-hydrogen is an equilibrium mixture of ortho and para hydrogen.
The amount of ortho and para hydrogen varies with temperature as:
- At 0°K, hydrogen contains mainly para hydrogen which is more stable.
- At the temperature of liquefaction of air, the ratio of ortho and para hydrogen is 1:1.
- At the room temperature, the ratio of ortho to para hydrogen is 3:1.
- Even at very high temperatures, the ratio of ortho to para hydrogen can never be more than 3:1.
Applications of hydrogen
- Hydrogen gas as the clean fuel of the future – generated from water and returning to water when it is oxidized. Hydrogen-powered fuel cells are increasingly being seen as ‘pollution-free’ sources of energy and are now being used in some buses and cars.
- Cars are very important for this new technique, because a lot of the carbon-dioxide (CO2) pollution is coming from the emission of cars. If cars in the future are driving on hydrogen-gas instead of petrol, the emission will be vapor instead of pollution with CO2.
- Some car companies are developing the so called 'hybrid'-vehicles. Hybrid-vehicles are cars which are driving on water.
- The low density of hydrogen made it a natural choice for one of its first practical uses – filling balloons and airships.
- It is also used to remove sulfur from fuels during the oil-refining process.
Related Keywords
 SureDen
SureDen