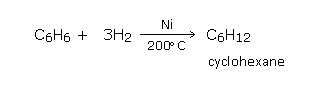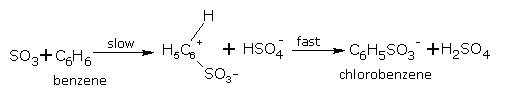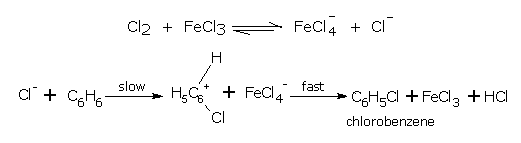Reactions of Benzene
Addition reactions of benzene
Benzene gives some addition reactions. Some typical reactions are:
Addition of hydrogen
Benzene on reduction with hydrogen under pressure in the presence of finely divided nickel at 200°C, gives an addition product hexahydrobenzene (cyclohexane).a
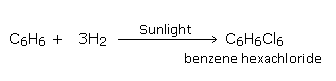
Addition of chlorine
Chlorine adds on to benzene at its boiling point, in the presence of bright sunlight, to give hexachloride.
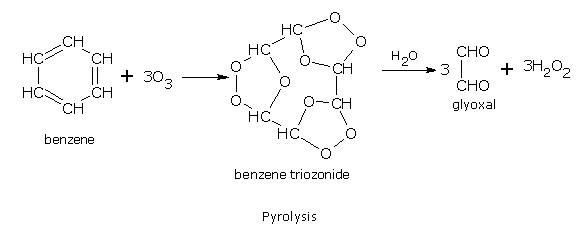
Addition of ozone
Benzene reacts slowly with ozone to form triozonide. Triozonide on hydrolysis with water gives glyoxal.
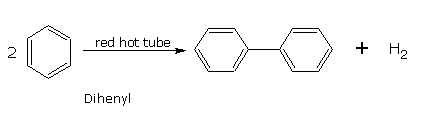
Benzene when passed through a red hot iron tube gives diphenyl.
Electrophilic substitution reactions of benzene
Benzene gives substitution reactions as described below.
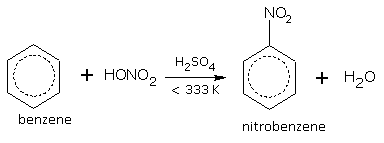
Nitration
When benzene is heated with concentrated HNO3 in presence of concentrated sulphuric acid at 333 K, we get nitrobenzene.
[A mixture of conc. HNO3+conc.H2SO4 is called as 'Nitrating Mixture'.]
At a higher temperature, another H-atom can be replaced by NO2 group to produce dinitrobenzene.
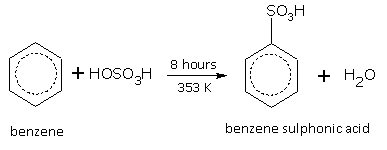
Sulphonation
When benzene is heated with concentrated sulphuric acid at 353 K for 8 hours, benzene sulphonic acid is formed.
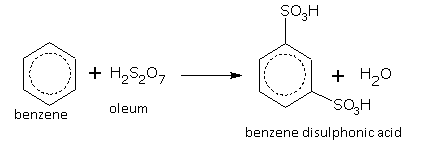
Oleum gives m-disulphonic acid.
Mechanism
The accepted mechanism for sulphonation is given below:
2H2SO4 ⇌ H3O+ + HSO-4 + SO3
C6H5SO-3 + H3O+ ⇌ C6H5.SO3H + H2O
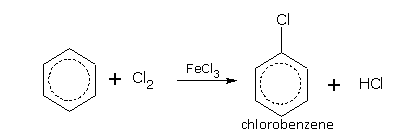
Halogenation
In halogenations, the nature of the products depends upon the reaction conditions.
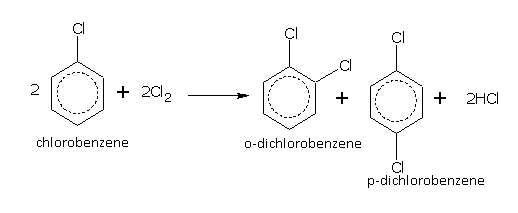
Benzene in the presence of a halogen carrier e.g., FeCl3 or iodine, reacts with chlorine and bromine even in dark and at room temperature to give mono- and di-halo derivatives,
Bromine reacts with benzene to form bromo derivatives. Iodine does not react with benzene.
Mechanism
The accepted mechanism of substitutional halogenation of aromatic compounds is described below.
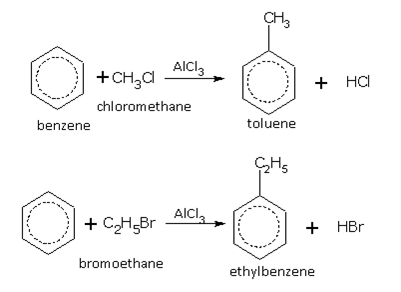
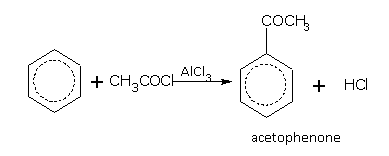
Friedel-Crafts reaction
This reaction is used for introducing an alkyl (R-) or acyl (RCO-) group into the benzene ring. Benzene, in the presence of anhydrous aluminium chloride (AlCl3), reacts with substances such as haloalkane (RX), alcohol (ROH) and acid chlorides (RCOCl) etc., to give the corresponding derivative of benzene. Some examples of alkylation are,
These two reactions are called alkylation of benzene.
Some examples of acylation are,
Introduction of COCH3 group in benzene ring is called acylation of benzene.
Directive influence in arenes
During the formation of monosubstituted products in benzene as all the six hydrogens are equivalent any of the six positions can be occupied. But, when the monosubstituted product is to be converted into disubstituted one, the existing substituent present in the ring directs the incoming group to a particular position. This is referred to as directive influence of the group. Depending upon their directive influence, various groups/substituents/functional groups can be divided into two categories as described:
The substituents or groups, which direct the incoming group to ortho and para positions are called ortho and para directing groups. For example:
-CH3,-C2H5-, -Cl, -OH, -Br, -NH2, -NHR, -NR2, -NHCOCH3, -OCH3 etc.
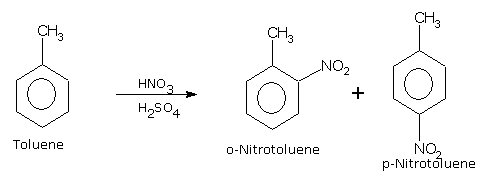
If we carry out nitration of toluene, the mixture of ortho and para nitrotoluenes are formed.
These groups (except halogens) increase the electron density at the ring through resonance effect. Thus the reactivity of benzene ring towards electrophilic substitution reactions increases.
The substituents or groups which direct the incoming group to meta position are called meta directing groups. For example:
-NO2, -CN, -CHO, -COR, -COOH, -COOR, -SO3H, etc.
The nitration of benzoic acid produces m-nitrobenzene.
These groups withdraw the electrons from benzene ring through resonance effect, reducing the electron density at the benzene ring. They decrease the reactivity of benzene ring towards electrophilic substitution reaction and make it less susceptible to the electrophilic attack.
Oxidation
Benzene is slowly oxidised by chromic acid, acidified KMnO4 etc., to CO2 and H2O.
When benzene vapours mixed with air are passed over V2O5 (vanadium pentoxide) at 400°C, maleic anhydride is obtained.
Oxidation of alkyl sidechain
The alkyl sidechain in the molecule of an arene can be oxidised under different conditions.
With hot acidified KMnO4 or K2Cr2O7, the sidechain gets oxidised to COOH group irrespective of the length of the sidechain. For example,
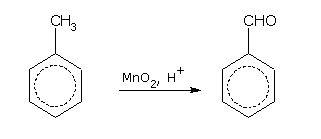
With weak oxidising agents such as acidic manganese dioxide (MnO2) or chromylchloride (CrO2Cl2), the side chain is oxidised to aldehyde (-CHO) group.
 SureDen
SureDen