Preparation of Benzene
The simplest arene is benzene (C6H6). Benzene is a typical arene and it gives all the typical reactions of arenes. Benzene was first isolated by Faraday (1825) from the cylinders of the compressed illuminating gas obtained from natural sources. In 1845, Hoffmann isolated benzene from coal-tar. Coal-tar is the chief source of benzene.
Preparation of Benzene
Benzene and its homologues may be prepared by the following methods.
By the decarboxylation of sodium benzoate
This method is used in the laboratory to obtain benzene. Sodium benzoate is heated with soda-lime and when it gets decarboxylated (removal of carbon dioxide) benzene is obtained.
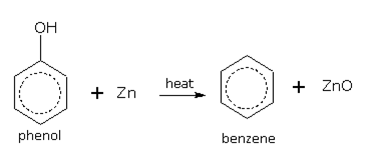
By heating phenol with zinc
When phenol vapours are passed over heated zinc dust, benzene is formed.
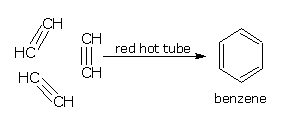
By the polymerization of ethyne (acetylene)
When ethyne (acetylene) is passed through a red hot copper tube, it polymerises to benzene.
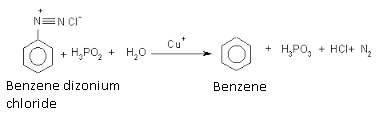
By the reduction of benzene diazonium chloride
Benzene is formed on the reduction of benzene diazonium chloride with sodium stannite or hypophosphorus acid.
By the hydrolysis of sulphonic acid
Benzene sulphonic acid on hydrolysis with superheated steam gives benzene.
benzene sulphonic acid benzene
 SureDen
SureDen