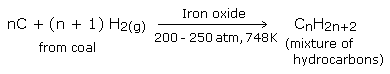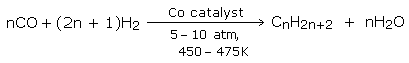Hydrocarbon form Coal
Aliphatic hydrocarbon form coal
The petrol obtained artificially from coal as a mixture of alkanes resembling petroleum like aliphatic hydrocarbon fuels is called synthetic petrol. Two important methods for producing synthetic petrol are the Fischer-Tropsch process and the Bergius process.
Bergius process
In this process, powdered coal is mixed with heavy oil and heated with hydrogen under high pressure (200-250 atm) at about 748 K in presence of iron oxide as catalyst.
The vapours on condensation give a liquid resembling crude oil. This is called synthetic petroleum, which on fractional distillation gives petrol (gasoline).
Fischer-Tropsch process
In this process, a mixture of water gas and hydrogen under pressure (5-10 atm) is passed over a cobalt catalyst at 450 - 475 K. The water gas required is obtained by passing steam over red-hot coke.
C (red hot) + H2O(g) → CO + H2water gas
The product so obtained is fractionally distilled to obtain petrol, middle oil and heavy oil. Further hydrogenation of the middle oil fraction then produces petrol.
Aromatic hydrocarbon form coal
Aromatic compounds from coal
Coal is a complex mixture of hydrocarbons. It also contains some organic compounds containing nitrogen and sulphur in small amounts. It can be approximated to the formula (C3H4)n.
Coal is a hydrogen-deficient substance. It is assumed that the basic structure of coal is probably built up of a large number of interlocked benzene rings, upto thirty, in bituminous/anthracite coal. Hydrogen is present in the aliphatic side chains. Bituminous coal has to be thermally decomposed, for obtaining organic compounds from coal. This process is called destructive distillation or 'pyrolysis' of coal.
Destructive distillation of coal
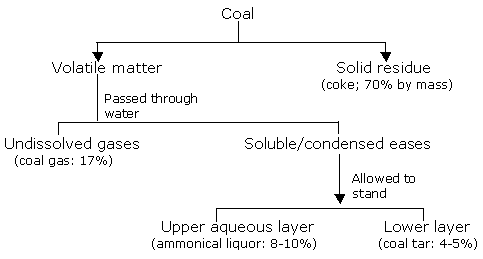
Destructive distillation of coal occurs when coal is heated in the absence of air, at high temperatures. To achieve this, coal is heated in the absence of air in iron retorts, wherein the volatile material evolved is collected as distillate/condensate. The process can be carried out under different temperature conditions, depending upon the nature of the products required.
Low temperature pyrolysis
Coal when heated in the absence of air at about 723 - 973 K gives a soft solid, smokeless coke called coalite. The yield of coal tar and coal gas is doubled here. Coal tar so obtained contains more of aliphatic compounds.
High temperature pyrolysis
In this process, coal is heated in the absence of air to about 1273 - 1473 K. The major products of high temperature pyrolysis are,
Coal
The yield per ton of coal on destructive distillation at higher temperature is,
Coke Coal gas Coal tar Ammonium sulphate Light oil 680 kg 30 m3 15 - 22 kg 1 kg 5 - 6 kg
The coal tar obtained is a heavy viscous almost black liquid due to carbon content with an unpleasant odor. It is a valuable by-product in the destructive distillation of coal. Its composition depends upon the nature of coal used during distillation. However, coal tar generally contains the following compounds: Acidic compounds (Phenol and cresols etc.), basic compounds (Pyridine, etc.) and Neutral compounds (Benzene, toluene, naphthalene and anthracene etc.)
Fractional distillation of coal tar
The outgoing hot vapors from the main iron still preheat coal tar obtained from the destructive distillation of coal, in a pre-heater. In this process, coal tar loses most of the water it contains, along with some low boiling hydrocarbons. It is then sent to the main iron still (a side retort) heated directly in a furnace. The vapors are condensed to obtain various fractions at different temperatures. Various fractions obtained during fractional distillation of coal-tar are given below.
Various fractions obtained from the fractional distillation of coal tar
|
Fraction |
Temperature Range |
Name of the fraction |
Constituents |
% (v/v) |
|
|
° |
K |
||||
|
1 2 3 4 5 |
up to 170 170 – 230 230 – 270 270 – 360 |
up to 443 443 – 503 503 – 543 543 – 633 |
Light oil Middle oil Heavy (creosote) oil Anthracine (green) oil Pitch residue in still |
Benzene, Toulene, Xylene Phenol, Cresol, Napthalene Cresol, Napthalene, Naphtol Anthracene Carbon |
2.25 7.5 16.5 12 56 |
Quality of gasoline, octane member and gasoline additive
The explosive nature of a hydrocarbon is determined by its volatility. The volatility of any liquid depends upon the temperature.
'The flash point of any liquid is the lowest temperature at which a liquid hydrocarbon gives off enough vapor to form an explosive mixture with air'.
The flash point of any liquid hydrocarbon is so adjusted that it remains safe under the conditions of its use. For example, the minimum flash point permitted in India is 44°C, while in France it is 33°C and in Britain it is 22°C.
Knocking of fuels
An internal combustion engine works with a system of pistons. A mixture of air and petrol vapor, is drawn from the carburetor into the cylinder in the down-stroke of the piston. In the upstroke phase, the mixture is compressed. The ratio of the initial volume to final volume is called the compression ratio. At the end of the upstroke of the piston, a spark ignites the compressed air-petrol (gasoline) mixture. As the gases burn, they expand and the flame front moves in a smooth manner and supplies power to the engine.
To achieve maximum efficiency of the engine, a high compression ratio of about seven to eight is required. However the increase in the compression ratio, results in the burning of petrol-air mixture in an explosive manner, that produces a metallic sound. This sound is called knocking, and indicates inefficient performance of the gasoline. High compression ratio engines also require less fuel, so petrol having less knocking tendencies are very valuable.
It has been found that the knocking tendency of the fuels falls off with the nature of the fuel as follows.
Straight chain alkanes > Branched chain alkanes > Alkenes
Knocking may also be prevented or minimized by adding compounds such as tetraethyl lead (TEL) to gasoline. Such compounds are called anti-knocking agents. To prevent the deposition of lead inside the cylinder, dibromoethane is added to the gasoline.
 SureDen
SureDen