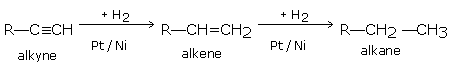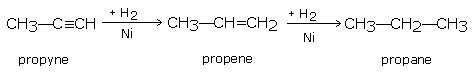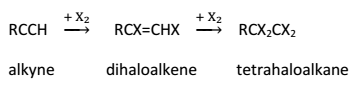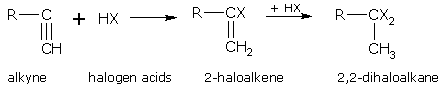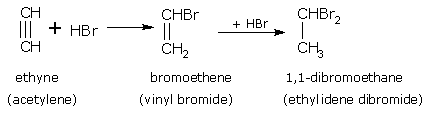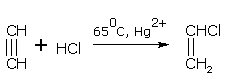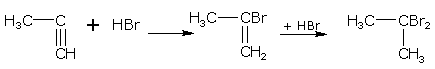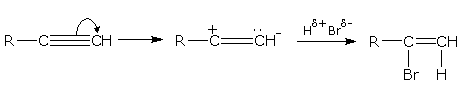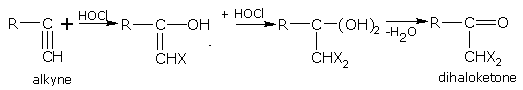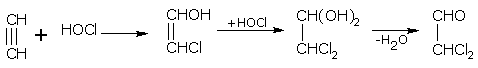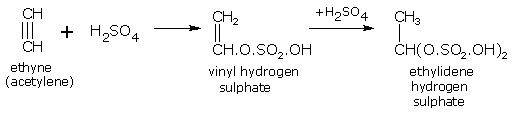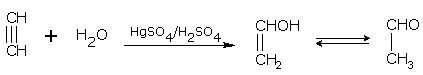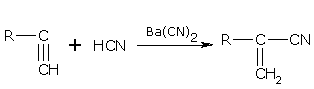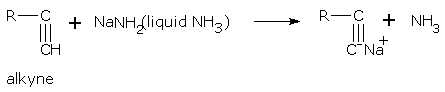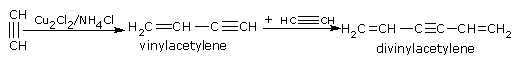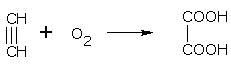Chemical Properties of Alkynes
Alkynes contain a triple bond (≡). A triple bond has one and two bonds.
Some characteristic reactions of alkynes are,
Combustion
Alkynes burns in air or oxygen with smoky flame.
CnH2n-2 + ((3n – 1)/2)O2 → n CO2 + (n – 1) H2O
Electrophilic addition reactions
Carbon-carbon triple bond, C=C, is a combination of one and two bonds. Alkynes give electrophilic addition reactions as they show reactivity due to the presence of bonds. This property is similar to alkenes but alkynes are less reactive than alkenes towards electrophilic addition reactions due to the compact CC electron cloud. Some typical electrophilic addition reactions given by alkynes are:
Addition of hydrogen
An alkyne reacts with hydrogen in the presence of catalyst (Pt or Ni) at 250°C, first forming alkenes and finally alkane.
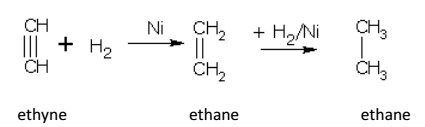
For example, ethyne gives ethane in two steps.
Ethane is obtained in good yields if hydrogenation is done with a calculated amount of hydrogen in the presence of palladium or barium sulphate.
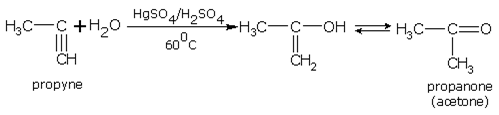
Propyne gives,
Addition of halogens
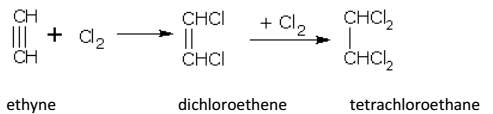
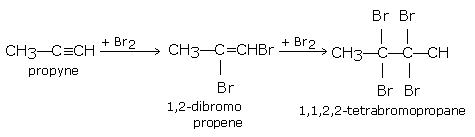
Alkynes react with halogens (Cl2 or Br2) in the dark, forming dihaloalkenes first and finally tetrahaloalkanes. The reaction gets accelerated in the presence of light or halogen carriers.
For example, ethyne (acetylene) with chlorine gives,
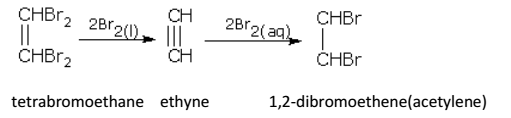
Dilute bromine water with ethyne gives dibromo, while liquid bromine gives tetrabromo derivative.
propyne gives,
The order of reactivity is Cl2 > Br2 > I2.
Addition of halogen acids
Alkynes reacts with halogen acids according to the Markownikoff's rule i.e. the carbon atom carrying the least number of hydrogen atoms will have the negative part of the addendum attached to it.
For example, ethyne (acetylene) with HBr gives,
With diluted HCl at 65°C and in the presence of Hg2+ (mercuric ion) ethyne gives vinyl chloride.
vinyl chloride
Propyne gives
propyne 2-bromopropene 2,2-dibromopropane
The rate of addition of halogen acids follows the order, HI > HBr > HCl
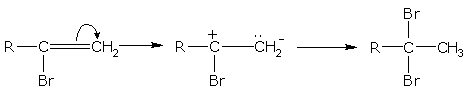
Mechanism
Addition of hypochlorous acid
Alkynes react with hypochlorous acid according to the Markownikoff's rule.
For example, ethyne with HOCl gives,
dichloroethanal
In the presence of peroxides the addition of HBr takes place according to the anti-MarkowniKoff's rule.
Addition of sulphuric acid
Alkynes add up two molecules of sulphuric acid. For example, ethyne gives
Nucleophilic addition reactions
Alkynes also give the following nucleophilic addition reactions.
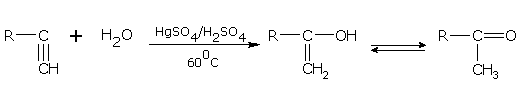
Addition of water
In the presence of sulphuric acid (42%) and 1 % mercuric sulphate at 60°C, alkynes add on one water molecule to give aldehydes or ketones. For example,
alkyne ketone
Ethyne gives ethanal and propyne gives acetone.
ethyne (acetylene) ethanal (acetaldehyde)
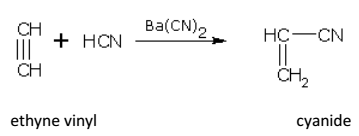
Addition of HCN
Alkynes add one molecule of HCN in the presence of Ba(CN)2. For example,
Ethyne gives
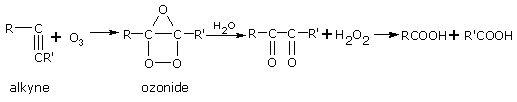
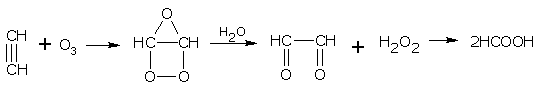
Addition of ozone
Ozone adds up across the triple bond to give ozonides. After hydrolysis, ozonides give diketones and carboxylic acids.
Ethyne gives glyoxal and formic acid,
glyoxal formic acid
Substitution reactions
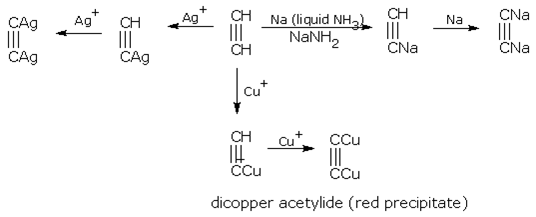
Due to their acidic nature, alkynes form metallic salts called alkynides e.g., sodium, silver and copper(ous) salts. Examples are,
Ethyne (acetylene) has two acidic hydrogen atoms, hence it finally gives dimetal salts.
Acidic hydrogen in 1-alkynes
Hydrogen atoms in ethyne and 1-alkynes, linked to the carbon atom having a triple bond on it, are acidic in nature. For example, ethyne (acetylene) is a weak acid: weaker than water but stronger than ammonia. This may be explained as follows:
The -electrons are more weakly bound than electrons. Thus, in those compounds containing carbon-carbon double or triple bonds, the electron density around such carbon atoms will be lesser than the carbon atoms linked only through bonds. Thus, electronegativity of differently hybridized carbon atoms will follow the order,sp > sp2 > sp3
i.e., the electronegativity will increase with the s character in the hybrid orbitals. This increase in the electronegativity of an alkyne carbon, (relative to the carbon atoms in alkenes and alkanes) will polarize the C-H electron bond towards carbon and facilitate the release of proton(s). Accordingly the acid strength of hydrogens will follow the order,Alkynes > Alkenes > Alkanes.
The stabilities of the anion left after the removal of proton, i.e. carbanions follow the order,
RC ≡ C- > RCH = CH- > R-CH2-CH2-
Thus, the acid strength follows the order, HC CH > H2C = CH2 > H3C-CH3
Compared to the organic acids e.g.. ethanoic acid (CH3OOH), ethyne is about 1020 time less acidic, while ethane is 1040 times less acidic.
Polymerization
On heating alkynes undergo polymerization in the presence of catalyst. The nature of products depends upon the conditions. For example,
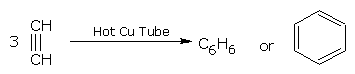
• When ethyne (acetylene) is passed through a hot copper tube, it polymerizes to benzene.
ethyne benzene benzene
• When passed through a solution of cuprous chloride in ammonium chloride, ethyne undergoes linear polymerization.
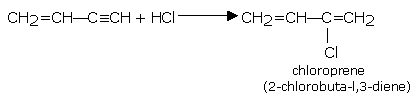
• Vinyl acetylene with hydrogen chloride gives chloroprene (2-chlorobuta-1,3-diene), which readily polymerizes to give neoprene (a synthetic rubber).
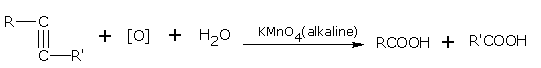
Oxidation
Oxidation of alkynes gives mono or dicarboxylic acids.
For example,
• Alkaline KMnO4 oxidises ethyne to oxalic acid.
oxalic acid(ethanedioic acid)
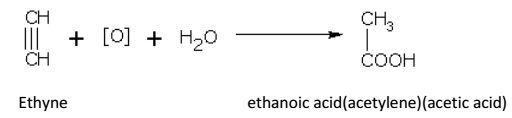
• With chromic acid, ethyne gives acetic acid.
Homologues of ethyne on oxidation with alkaline KMnO4 give mixture of acids. During oxidation, rupture takes place at the triple bond.
 SureDen
SureDen