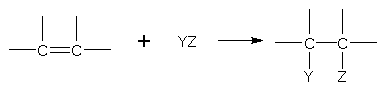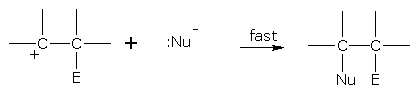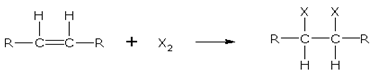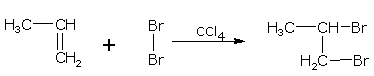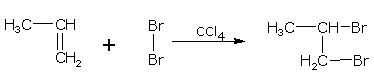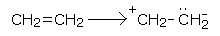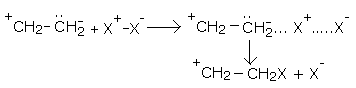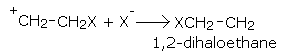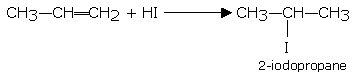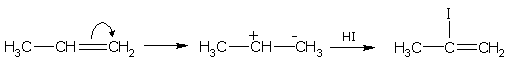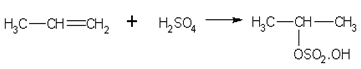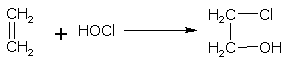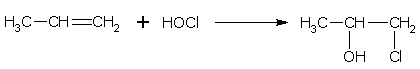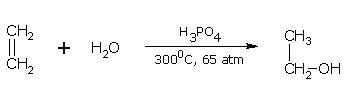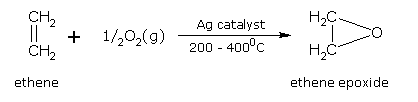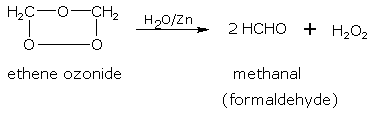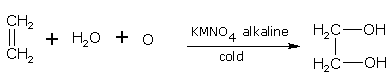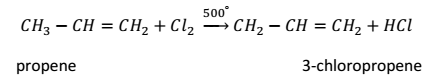Chemical Properties of Alkene
Chemical properties of Alkene
Alkenes are more reactive than alkanes due to the presence of a double bond. The carbon-carbon double bond consists of a strong bond and a weakp bond. The typical reactions of alkenes involve the breaking of this weakerp bond, viz., and formation of two sigma (s) bonds.
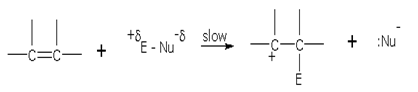
Such reactions are called addition reactions and are initiated by an electrophile, proceeding through ionic mechanism. However, some addition reactions proceed through free-radical mechanism.
Higher alkenes contain a long chain of carbon. That part of the chain that forms an alkane-like structure (consisting of C-C bonds), may undergo substitution reaction as also shown by alkanes. Some characteristic reactions shown by alkenes are described below:
Combustion
Alkenes, like alkanes, are highly combustible. Alkenes burn with a luminous flame to give carbon dioxide and water. The flame becomes luminous because of the higher carbon content of alkenes than alkanes. Their combustion reactions are exothermic.
CnH2n + (3n/2) O2 (g) ⟶ nCO2 + nH2O + heat
Due to the luminosity of the flame, the lower alkenes may be used as illuminants.
Addition reactions
The p electrons of the carbon-carbon-double bond are available to an electrophile (any species seeking electrons). Thus, the addition reactions shown by alkenes are in fact electrophilic addition reactions.
addition product
Some addition reactions proceed through free-radical mechanism.
Addition of hydrogen
Alkenes add hydrogen in the presence of platinum or nickel catalyst, to form alkanes. The reaction termed as hydrogenation, is an exothermic reaction.
CnH2n+ H2 CnH2n+2 + heat
This is known as Sabatier-Senderens reduction.
CH2=CH2 + H2 CH3-CH3 + 132.2 kJ
ethene ethane
Addition of halogens
Alkenes react with halogens to form dihaloalkanes. The order of reactivity is, chlorine > bromine > iodine. Simply mixing together the two reactants, usually in an inert solvent like carbon tetrachloride, best carries out the reaction.
Alkene dihaloalkane
ethene 1,2-dibromoethane
propene 1,2-dibromopropane
Addition of bromine is useful for the detection of the carbon-carbon double bond. When a 5% solution of bromine in carbon tetrachloride is added to an alkene, it gets decolorized. This indicates the presence of a double bond in the molecule. This test is called 'bromine test'.
Mechanism of halogen addition
The addition of halogen across the double bond takes place through the following steps. Taking an example of ethene,
• Ethene molecule undergoes electromeric effect
• Due to its close proximity to the carbon-carbon double bond, the non-polar halogen molecule gets polarized
X – X ⟶ X+ - X-
• The polarized halogen molecule forms a transition-state complex with ethene molecule.
• The X- ion attaches itself to the positively charged carbon.
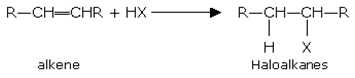
Addition of halogen acids
Alkenes with concentrated aqueous solution of halogen acids give haloalkanes. The order of reactivity is,HI > HBr > HCl
For example:
Ethene gives
Ethane haloethane
2-butene with HBr gives
H3C — CH = CH — CH3 + HBr ⟶ H3C — CH2 — CH(Br) — OH3
2-Butene 2-bromobutane
Thus, symmetrical alkenes give only one product, due to the equivalence of the two carbon atoms (the H and X may add to the molecule in any way).
In asymmetrical alkenes, the addition of a halogen acid takes place in a manner where by the halogen atom (the negative part of the molecule to be added) adds to the carbon atom, which has lesser number of hydrogen atoms on it. For example, in the case of propene, the product obtained is 2-iodopropane and not 1-iodopropane.
The I being negative part of the added molecule, goes to the carbon number 2 because it has only one H-atom on it. (lesser number of H-atom)
This rule of the addition of halogen acids to an asymmetrical alkene is known as Markownikoff's rule (1869).
Markownikoff's rule
This is an empirical rule but it may be explained theoretically on the basis that the addition occurs by a polar mechanism. For example, the addition of HI to propylene. Since a methyl group is electron-repelling, the propylene molecule is polarized as follows.
2-iodopropane
Hence, the proton of the hydroiodic acid gets attached to the negatively charged carbon and the iodide ion to the positive carbon.
Peroxide effect
The mode of addition of hydrogen bromide to unsymmetrical alkenes in the presence of oxygen and peroxides is contrary to Markownikoff rule. This addition of HBr to unsymmetrical alkenes against the Markownikoff's rule is known as peroxide effect, or anti-Markownikoff's rule.
For instance, the reaction of propene with HBr in the presence of peroxides, forms 1-bromopropane instead of 2-brompropane.
CH3-CH=CH2 + HBr → CH3-CH2-CH2Br1 -bromopropane
The mode of addition of hydrogen chloride or hydrogen iodide is not affected by the presence of peroxides.
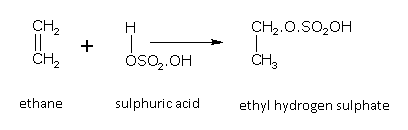
Addition of sulphuric acid
In accordance to Markownikoff's rule alkenes readily add concentrated sulphuric acid to form alkyl hydrogen sulphates. For example,
Ethene gives,
Propene gives,
Iso-propyl hydrogen sulphate
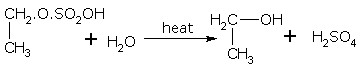
An alkyl hydrogen sulphate on boiling with water gives the alcohol and sulphuric acid. Alcohols are prepared from alkenes obtained from the cracking of petroleum. For example,
ethyl hydrogen sulphate ethanol
Addition of hypohalous acids
Hypohalous acid (HOX) in accordance with the Markownikoff's rule, add to the molecule of an alkene at the double bond. For example,
ethene hypochlorous acid 2-chloroethanol(ethylene chlorohydrin)
propene 1-chloro-2-propanol(propylene chlorohydrin)
Addition of water (Hydration of alkenes)
Water molecule adds to an alkene molecule across the double bond in the presence of dilute acids and a catalyst. For example, ethane gives ethanol when a mixture of ethene and steam is passed over phosphoric acid and silica under a pressure 65 atm, and at 300C.
ethene ethanol
Addition of oxygen
Lower alkenes are mixed with air and passed under pressure over a silver catalyst at 200-400°C. This gives epoxides by adding one atom of oxygen across the double bond. The epoxides so obtained are used in detergents.
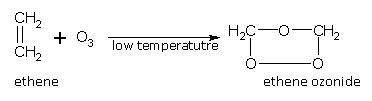
Addition of ozone
Ozonides are formed when alkenes add a molecule of ozone across the double bond. For example, ethene gives ethene ozonide.
Ozonides on hydrolysis with water in the presence of a reducing agent give aldehydes.
The oxidation of alkenes by ozone followed by decomposition of the formed ozonide with water, is termed as 'ozonolysis'. The nature of the products (aldehydes and ketones) formed due to ozonolysis depends upon the location of the double bond in the parent alkene. Therefore, this reaction provides a very convenient way of locating the position of the double bond in any molecule.
As in the above example, the only product formed upon the hydrolysis of ethene ozonide is formaldehyde (containing one carbon unit each) hence the double bond has only one carbon unit on either side.
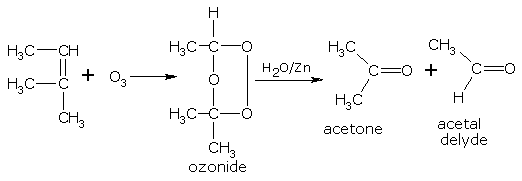
In the following example,
The products of ozonolysis are, acetone (3 carbon unit) and acetaldehyde (2 carbon unit).
It implies the location of the double bond being between two carbon chains of 2 carbon and 3 carbon atoms.
Oxidation
Alkenes can be readily oxidized, but the nature of the products depends upon the oxidizing agent used.
4">
With cold, alkaline KMnO4
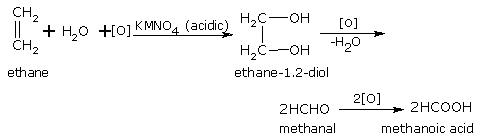
When alkenes are oxidised with cold, alkaline KMnO4, dihydroxy compounds (diols or glycols) are formed. The KMnO4 gets decolorized. This reaction is therefore, used as Bayer's test for unsaturation (the presence of double or triple bonds) in any molecule.
Ethene gives ethane-1,2-diol.
ethane ethane-1,2-diol
4-Or-K2Cr2O7">
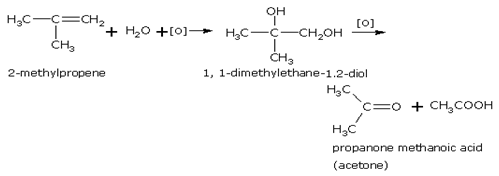
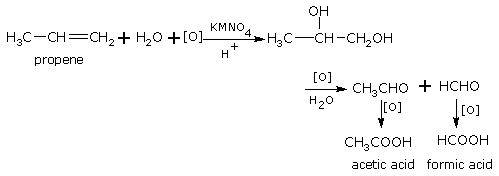
With acidified KMnO4 Or K2Cr2O7
Acidified potassium permanganate (or potassium dichromate) oxidises the dihydroxy compound so produced in reaction to ketone and/or carboxylic acid. For example,
Substitution reactions
At elevated temperatures (500°C), higher alkenes give substitution products with chlorine. For example,
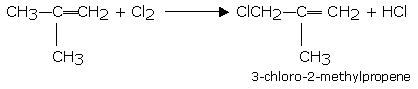
Branched-chain alkenes give substitution reaction easily. For example isobutene gives substitution product with chlorine even at room temperature.
 SureDen
SureDen