Process involving physical equilibrium
The process involving physical equilibrium are:
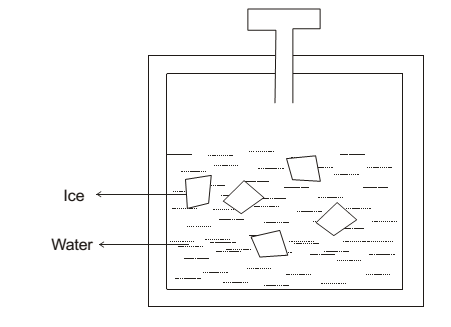
Place some ice piece in contact with water at 273K & at normal atmospheric pressure. In a closed insulated flask. It is observed that there is no change in the mass of ice piece & water. It doesn’t mean that melting of ice stop at this pt. but rate of melting of ice = rate of freeing of water
2)
Take a small amount of water in a vessel consisted with a manometer at room temperature after some time we observe that the level of the mercury start rising in right limb while start falling in the left limb & become constant after sometime. It doesn’t mean that process stop at this stage or rate of evaporation = rate of condensation & the stage is known as liquid- vapour equilibrium.
⟹ At this stage, the pressure exerted by the vapour in equilibrium with the liquid at a particular temperature is called equilibrium vapour pressure of the liquid.
3) :- If some solid iodine is placed in a closed container, the container start filing with violet vapour, it doesn’t mean that evaporation of iodine stop but rate of vaporization is equal to rate of solidification.
4):-If we add some sugar in water, it will dissolve & this process is known as dissolution. If we keep on adding more sugar to this solution, it will go on dissolving until the solution becomes saturated & no more sugar dissolve. It doesn’t mean that process of dissolution stops but rate of dissolution of sugar is equal rate of crystallization of sugar.
 SureDen
SureDen