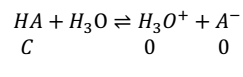Degree of dissociation
Degree of dissociation (degree of ionization):-
It may be defined as the fraction of total no. of molecules of an electrolyte which ionizes into ion.
If value of ∝ = 0.99, it is strong electrolyte.
Let us consider weak acid
K = C∝2
Hence, Ostwald’ dilution law states the degree dissociation of any weak electrolyte is inversely proportional to the square root of molar concentration & directly proportional to the square root of dissociation constant or ionization constant of acid.
Related Keywords
 SureDen
SureDen