Bronchid concept of acid and base
- Bronchid’s concept of acid & base:-
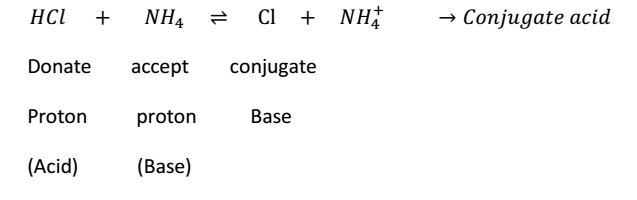
According to Bronchid’s concept, an acid a is a substance which can donate proton H+, while a base is a substance which can accept proton H+ ion hence an acid is a proton-doner, & base is a proton acceptor.
HCl & Cl- Conjugate- acid – base pair
NH3 & NH4+ are conjugate acid base pair.
Hence, the pair of acid & base which are formed from each other by the gain or loss of a proton are could conjugate acid-base pair.
Acid = Conjugate base + H+
Base = Conjugate acid – H+
Limitation of Bronchid’s Concepts:-
- Like Arrhenius concepts, it doesn’t explain the acidic basic nature of oxide like CO2, SO2 etc.
- Substances like BF3 or AlCl3 act as acid but not proton donor.
Related Keywords
 SureDen
SureDen