Modern Periodic Table
Modern periodic law:- It is based upon Moseley’s saying that physical and chemical properties of elements are function of their atomic number.
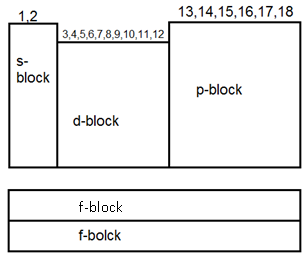
Characteristics of Modern Periodic Table (M.P.T.)
(1) There are 18 groups and T periods.
(2) It has four blocks ( s, p, d, f )
S- block element:- (a) General Electronic configuration ns1-2
(b) It includes alkali and alkaline earth metals.
(C) They form mainly ionic compounds.
(d) Low ionization energy and low melting and boiling point
(e) highly electropositive elements.
(f) It is placed to the extreme left side of the P.T(periodic table)
p- block elements:- (a) general electronic configuration is ns2 np1-6
(b) It is placed to extreme right side of Periodic Table
(C) They form covalent bonds.
(d) It includes metals, non-metals and metalloids.
d- block elements:- (a) General eΘ configuration is (n-1)d1-10 ns0-2
(b) It includes those elements are those in which the last electron goes to d-subsheels [Penultimate shell (II last shell)]
(c) They form colored complexes.
(d) They show variable oxidation states and catalytic properties.
f- block elements:- (a) Those elements in which the last eΘ goes to anti-penultimate (III last shell).
(b) General Electronic Configuration (n-2)f1-14 (n-1)d0-1 ns2
(c) It includes heavy metals.
(d) Form colored complexes.
(e) also known as lave earth metals.
(f) Most of the f-block elements are radioactive in nature.
(3) Group I → Alkali Metals.
Group II → Alkaline Earth Metals
Group 15 → Pnicogens (Suffocating)
Group 16 → Chalcogens (Ore forming)
Group 17 → Halogens (Salt forming)
Group 18 → Noble Gases.
(4) Tanthanoids and Actenoids (f-block elements) are kept at the bottam of periodic table because there properties don’t match v the other elements of the main body f the periodic table.
(5) S- and p- block elements are known as representative elements.
IUPAC Naming of elements v atomic Number more than 100 (Super heavy elements).
|
Zero(0) |
Nil |
N |
|
1 |
Un |
U |
|
2 |
Bi |
B |
|
3 |
Ti |
T |
|
4 |
Quad |
Q |
|
5 |
Pent |
P |
|
6 |
Hex |
H |
|
7 |
Sept |
S |
|
8 |
Oct |
O |
|
9 |
Enn |
E |
Suffix (icem)
Question:- Write down the IUPAC name of the following and symbol also.
Ans.:- Name Symbol
111 unununium uuu
101 unnilunium unu
120 unbinilium ubn
117 ununseptium uus
118 ununoctium uuo
The position of elements v atomic No.
114 is 14group
117 is 17group
118 is 18group
119 is I group (8th period)
120 is II group (8 period)
121 has no position, last electron will go to 5g subshell because in our P.T. There is no g beak
Important first period has two elements it involves the filling of is subshell.
Second period has 8 eΘ it involves 2s & 2p.
Third period has 8 elements - 3s 3p
Fourth period has 18 elements - 3d 4s 4p
Fifth period has 18 elements - 4d 5s 5p
See Periodic Table in any Chemistry book.
 SureDen
SureDen