Valence shell electron pair Repulsion Theory
Valence shell Electron pair Repulsion theory
VSEPR Theory => It was given by Sidgwick and Powell in 1940 and was developed by Nyholm
And Gillespie in1957.
Basic Assumptions are=>
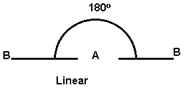
- The shape of molecule is linear if it contains only two elements.
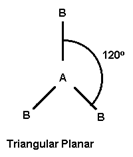
- The molecules containing 3or more atoms has one of the atoms as central atom to which others are attached.
- If central atom is attached to similar atoms and is surrounded by bond pain of Ä“ only then its structure will be symmetrical and molecule has regular geometry.
- If central atom linked to different atoms or is surrounded by bond pairs as well as lone pair of electrons. then molecule has unsymmetrical geometry and order of repulsion is Lone pair-Lone pair >L.P-B.P>B.P.-B.P
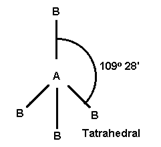
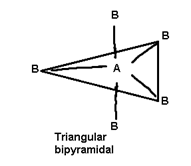
- Geometry of the molecule depends upon total number of electron pairs in molecule.
|
Type |
Bond pairs |
Shape of molecule |
Examples |
|
2 |
|
Be Cl2, HgCl2 |
|
3 |
|
Ef. B Cl3, Al Cl3 |
|
4 |
CH4, Si Cl4 |
|
|
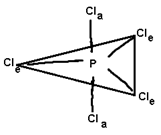
5 |
P Cl5, As F5 |
|
|
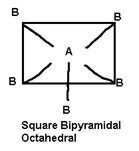
6 |
SF6, [Si F6]2-
|
Important Questions:-
1) CH4 is tetrahedral, not square planer why?
Ans:- Square planar structure Bond angle is 900 and in tetrahedral arrangement bond angle is 109o28’ which is more and bonds remains apart from each other to minimize repulsion and minimize stability.
2) PCl5 all the bond lengths (P-U) are not similar why?
PCl5. 2 types of bond lengths are present P-Claxial and P-Clequatorial P-Clax bonds are longer then P-Cleq
Shapes of molecules having bond Pair & Lone Pair
|
Molecule |
b.P |
L.P |
Shape & Geometry |
Remarks |
|
2 |
2 |
Bent or V shape |
? of presence of 2 lone pairs bond angle reduces from 109° 28’ to 104°50’ |
|
3 |
1 |
Pyramidal shape |
? of presence of 1 L.P b.A ↓ from 109°28’ to 107° |
|
4 |
1 |
|
L.P occupies equatorial position of ↓ repulsion |
|
3 |
2 |
|
|
|
4 |
2 |
|
|
Note: in water, 3 types of repulsion are present L.P – L.P > L.P – b.P > b.P – Bp & in NH3, 2 types of repulsions L.P – b.P > b.P – b.P
So in H2O bond angle is lesser then in NH3 (otherwise the expected shape of water and ammonia was tetrahedral)
VSEPR theory can’t explain the shapes of many other compounds.
 SureDen
SureDen