Limitations of Octect Rule
Limitations of octet Rule=> (i) Formation of compounds.
Involving hydrogen because H tends complete its duplet not octet
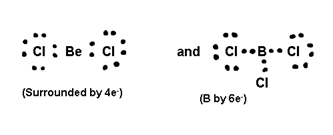
(ii) Formation of Be Cl2, BCl2 Compounds.
where octet of B and (surrounded by B is not completed 4) (B by 6
) i.e. these are
deficient compounds.
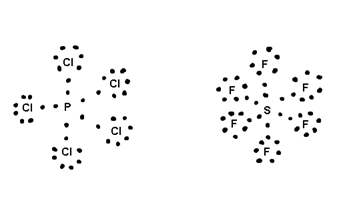
(iii) P cl2, SF6, IF, H2SO4 etc. have more then 8
i.e. these are Hypervalent compounds
Central atom surrounded by more than &
e.g.
(iv) Compounds of noble gases:-noble gases have already & in outermost shall they make compounds e.g. X eF2, X ef4 etc.
(v) e.g.
Related Keywords
 SureDen
SureDen