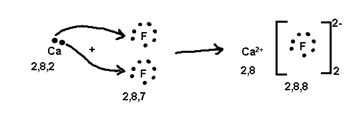
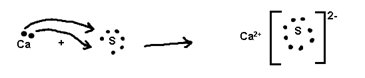
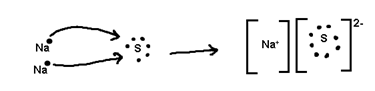
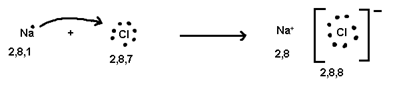Ionic or Electrovalent Bond
Ionic or Electrovalent Bond:-
The Number of electrons cost or gained during formation of tonic bond is known as electrovaleney.
Factors Affecting formation of Ionic bond=>
- Ionization Energy=> smaller the I.E. more easily electron is lost and higher will be case os formation of ionic bond. Eg- Alkali metels
- Electron gain Enthalpy (EGE)=> more -ve is EFE higher is case of formation of anion and more easily ionic bond is formed e.g. Halogens.
- Lattice Energy=> It’s the energy released when I mole of ionic Compd. Is produced from its constituents ions is the gaseous state.
- Higher the lattice Energy stronger is ions bond.
-Higher the charger present on ions ↑ is L.E.
- Smaller the Inter nuclear distance, ↑ is L.E.
The Number of electrons cost or gained during formation of tonic bond is known as electrovaleney.
Factors Affecting formation of Ionic bond=>
- Ionization Energy=> smaller the I.E. more easily electron is lost and higher will be case os formation of ionic bond. Eg- Alkali metels
- Electron gain Enthalpy (EGE)=> more -ve is EFE higher is case of formation of anion and more easily ionic bond is formed e.g. Halogens.
- Lattice Energy=> It’s the energy released when I mole of ionic Compd. Is produced from its constituents ions is the gaseous state.
- Higher the lattice Energy stronger is ions bond.
-Higher the charger present on ions greater is L.E.
- Smaller the Inter nuclear distance, greater is L.E.
Questions=> What is shape of LaF2
Ans:-- No shape ∵ Ionic bond is non-directional.
- Out of NaCl and Mgo, Mgo has greater L.E. greater Charges.
 SureDen
SureDen