Photoelectric Effect
Photoelectric Effect:
The phenomenon of emission of electrons from a metal surface when exposed to a light of suitable frequency is known as photoelectric effect.
The electrons emitted by a metal surface are known as photoelectrons. The current constituted by photoelectrons is known as photoelectric current.
Condition For photoelectric effect:
- Metal should have low Ionization i.e. mainly alkali metals are used.
- Metal should have low work function.
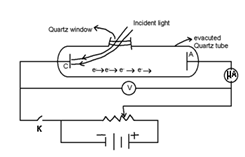
Experimental study of photoelectric effect:
- Effect of potential on photoelectric current:
When light of suitable frequency falls on the metallic plate C, photoelectrons are emitted. These electrons get accelerated towards the plate A which is at positive potential w.r.t. cathode & constitute the current called photoelectric current. For a fixed frequency and intensity of incident light, this photoelectric increases with the increase in the applied potential of plate A. And at a stage photoelectric current becomes maximum ; this maximum value known as saturation current. The saturation current will not increase with the increase is positive potential of plate A.
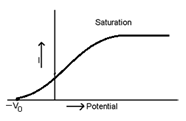
If the potential of plate A is decreased it attains negative potential with reference to plate C. The negative potential applied to plate A is increased to a certain value Vc for which no photoelectron reach the plate A. So at this potential, photoelectric current is zero.
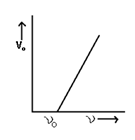
The minimum negative potential (Vo) applied to anode
For which photoelectric current becomes zero is called cut off potential or stopping potential.
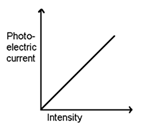
2.Effect of Intensity of light:
As the intensity of the incident light increases keeping the frequency constant; more and more photoelectrons are emitted by the cathode ‘C’ & hence photoelectric current increases linearly.
Intensity of incident light does not affect the stopping potential.
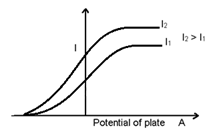
- Effect of Frequency of incident light on stopping potential:
The intensity of incident light is kept constant but the frequency is changed so that in each case the saturation current is exactly the same.
As V2 > V1
Vo∝ ν
Threshold Frequency:- The minimum value of frequency (νo) of the incident light below which photoelectric emission if not possible. It is also known as cut off frequency.
Law of Photoelectric Effect:
- The photoelectric current increases with the increase in the intensity of light.
- For photoelectric effect to be takes place a minimum frequency is required known as threshold frequency below which no photoelectric effect takes place.
- Above threshold frequency electrons are emitted from the metal surface & travel with kinetic energy which is independent of the intensity of light but depends on the frequency of light.
- The process of photoelectric emission is instantaneous i.e. it has been estimated that photoelectrons are emitted 10-9 sec
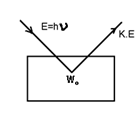
Einstein’s Photo-electric equation-particle nature of light:-
According to law of conservation of energy
E = Wo + K.E
hv = h ν o + K.E
K.E = h ν - hvo
K.E = h(ν – ν o)
Or 1/2 m ν 2 (ν – ν o)
1/2 m ν 2 = h (c/λ – c/λo)
1/2 m ν 2 = h c (1/λ – 1/λo)
As K.E = e V
e V = h c (1/λ – 1/λo)
Q. Photoelectric work function of a metal is 1 e V. Light of wavelength 3000A° falls on it, what is the velocity of the ejected photoelectrons?
Sol:- as Wo = 1 e V = 1.6 x 10-19 J
λ = 3000A° = 3000 x 10-10 m
or 3 x 10-7 m
as K.E = h c/λ – Wo
= ((6.6 x 10-34 x 3 x 108/3 x 10-7) – 1.6 x 10-19)
= 5.025 x 10-19 J
As K.E = 1/2 m v2
1/2 m v2 = 5.025 x 10-19
v = (5.025 x 10-19 x 2/9.1 x 10-31)1/2
v = 1 x 106 m/s (Ans.)
Q. The threshold wavelength of a metal is 360 nm. Find the maximum energy of the ejected photoelectrons by a radiation of 200 nm (Ans -2.76 e V).
 SureDen
SureDen