Isotopic forms of hydrogen
Isotopic forms of hydrogen:
Hydrogen is the most abundant element in the universe. About half the mass of sun and stars is due to hydrogen. Jupiter and Saturn planets consist mainly of hydrogen. It is common on earth in water, coal, petroleum, clay, animal and vegetable matter that constitutes 0.9% by weight of Earth's crust. It is the ninth element in order of abundance.
Isotopes of hydrogen
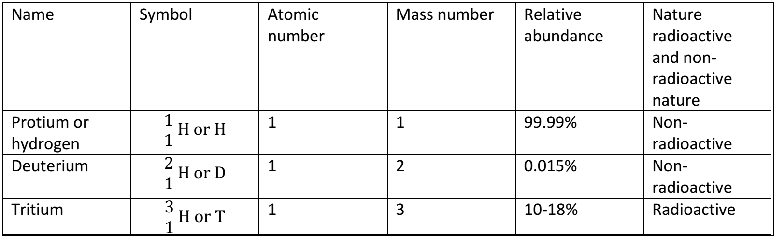
Isotopes are those forms of an elements, which have same atomic number but different mass numbers. The isotopes of hydrogen are protium, deuterium and tritium with mass number 1, 2 and 3 respectively. These isotopes differ only in the number of neutrons present in them.
Protium or ordinary hydrogen
It is the most abundant isotope of hydrogen. Its nucleus has one proton and no neutron (mass number = 1). It is represented as H.
Deuterium or heavy hydrogen
It is present in heavy water (D2O) and can be recovered by fractional electrolysis. Its nucleus has one proton and one neutron (mass number = 2). It is represented as D.
Tritium
It is rare due to the instability and radioactive nature of its nucleus. Its nucleus has one proton and two neutrons (mass number = 3). It is represented as T.
 SureDen
SureDen